Multisystem involvement, defective lysosomes and impaired autophagy in a novel rat model of nephropathic cystinosis
- PMID: 35137071
- PMCID: PMC9262394
- DOI: 10.1093/hmg/ddac033
Multisystem involvement, defective lysosomes and impaired autophagy in a novel rat model of nephropathic cystinosis
Abstract
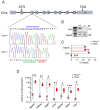
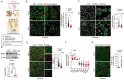
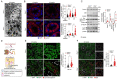
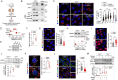
Recessive mutations in the CTNS gene encoding the lysosomal transporter cystinosin cause cystinosis, a lysosomal storage disease leading to kidney failure and multisystem manifestations. A Ctns knockout mouse model recapitulates features of cystinosis, but the delayed onset of kidney manifestations, phenotype variability and strain effects limit its use for mechanistic and drug development studies. To provide a better model for cystinosis, we generated a Ctns knockout rat model using CRISPR/Cas9 technology. The Ctns-/- rats display progressive cystine accumulation and crystal formation in multiple tissues including kidney, liver and thyroid. They show an early onset and progressive loss of urinary solutes, indicating generalized proximal tubule dysfunction, with development of typical swan-neck lesions, tubulointerstitial fibrosis and kidney failure, and decreased survival. The Ctns-/- rats also present crystals in the cornea, and bone and liver defects, as observed in patients. Mechanistically, the loss of cystinosin induces a phenotype switch associating abnormal proliferation and dedifferentiation, loss of apical receptors and transporters, and defective lysosomal activity and autophagy in the cells. Primary cultures of proximal tubule cells derived from the Ctns-/- rat kidneys confirmed the key changes caused by cystine overload, including reduced endocytic uptake, increased proliferation and defective lysosomal dynamics and autophagy. The novel Ctns-/- rat model and derived proximal tubule cell system provide invaluable tools to investigate the pathogenesis of cystinosis and to accelerate drug discovery.
© The Author(s) 2022. Published by Oxford University Press.
Figures



Similar articles
-
Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis.Hum Mol Genet. 2014 May 1;23(9):2266-78. doi: 10.1093/hmg/ddt617. Epub 2013 Dec 6. Hum Mol Genet. 2014. PMID: 24319100
-
Cystinosin-deficient rats recapitulate the phenotype of nephropathic cystinosis.Am J Physiol Renal Physiol. 2022 Aug 1;323(2):F156-F170. doi: 10.1152/ajprenal.00277.2021. Epub 2022 Jun 13. Am J Physiol Renal Physiol. 2022. PMID: 35695380
-
Cystinosis (ctns) zebrafish mutant shows pronephric glomerular and tubular dysfunction.Sci Rep. 2017 Feb 15;7:42583. doi: 10.1038/srep42583. Sci Rep. 2017. PMID: 28198397 Free PMC article.
-
Molecular Mechanisms and Treatment Options of Nephropathic Cystinosis.Trends Mol Med. 2021 Jul;27(7):673-686. doi: 10.1016/j.molmed.2021.04.004. Epub 2021 May 8. Trends Mol Med. 2021. PMID: 33975805 Review.
-
Hematopoietic Stem Cell Gene Therapy for Cystinosis: From Bench-to-Bedside.Cells. 2021 Nov 23;10(12):3273. doi: 10.3390/cells10123273. Cells. 2021. PMID: 34943781 Free PMC article. Review.
Cited by
-
Targeting oxidative stress-induced lipid peroxidation enhances podocyte function in cystinosis.J Transl Med. 2025 Feb 20;23(1):206. doi: 10.1186/s12967-024-05996-w. J Transl Med. 2025. PMID: 39980044 Free PMC article.
-
The pro-fibrotic role of autophagy in renal intrinsic cells: mechanisms and therapeutic potential in chronic kidney disease.Front Cell Dev Biol. 2024 Dec 11;12:1499457. doi: 10.3389/fcell.2024.1499457. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39723243 Free PMC article. Review.
-
Dietary supplementation of cystinotic mice by lysine inhibits the megalin pathway and decreases kidney cystine content.Sci Rep. 2023 Oct 12;13(1):17276. doi: 10.1038/s41598-023-43105-x. Sci Rep. 2023. PMID: 37828038 Free PMC article.
-
Unexpected mutations occurred in CRISPR/Cas9 edited Drosophila analyzed by deeply whole genomic sequencing.Heliyon. 2024 Mar 30;10(7):e29061. doi: 10.1016/j.heliyon.2024.e29061. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38596060 Free PMC article.
-
Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology.Nat Rev Mol Cell Biol. 2024 Mar;25(3):223-245. doi: 10.1038/s41580-023-00676-x. Epub 2023 Nov 24. Nat Rev Mol Cell Biol. 2024. PMID: 38001393 Review.
References
-
- van der Wijst, J., Belge, H., Bindels, R.J.M. and Devuyst, O. (2019) Learning physiology from inherited kidney disorders. Physiol. Rev., 99, 1575–1653. - PubMed
-
- Gahl, W.A., Thoene, J.G. and Schneider, J.A. (2002) Cystinosis. N. Engl. J. Med., 347, 111–121. - PubMed
-
- Nesterova, G. and Gahl, W. (2008) Nephropathic cystinosis: late complications of a multisystemic disease. Pediatr. Nephrol. (Berlin, Germany), 23, 863–878. - PubMed

