Sperm acquire epididymis-derived proteins through epididymosomes
- PMID: 35137089
- PMCID: PMC8971652
- DOI: 10.1093/humrep/deac015
Sperm acquire epididymis-derived proteins through epididymosomes
Abstract
Study question: Are epididymosomes implicated in protein transfer from the epididymis to spermatozoa?
Summary answer: We characterized the contribution of epididymal secretions to the sperm proteome and demonstrated that sperm acquire epididymal proteins through epididymosomes.
What is known already: Testicular sperm are immature cells unable to fertilize an oocyte. After leaving the testis, sperm transit along the epididymis to acquire motility and fertilizing abilities. It is well known that marked changes in the sperm proteome profile occur during epididymal maturation. Since the sperm is a transcriptional and translational inert cell, previous studies have shown that sperm incorporate proteins, RNA and lipids from extracellular vesicles (EVs), released by epithelial cells lining the male reproductive tract.
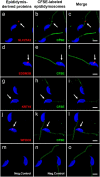
Study design, size, duration: We examined the contribution of the epididymis to the post-testicular maturation of spermatozoa, via the production of EVs named epididymosomes, released by epididymal epithelial cells. An integrative analysis using both human and mouse data was performed to identify sperm proteins with a potential epididymis-derived origin. Testes and epididymides from adult humans (n = 9) and adult mice (n = 3) were used to experimentally validate the tissue localization of four selected proteins using high-resolution confocal microscopy. Mouse epididymal sperm were co-incubated with carboxyfluorescein succinimidyl ester (CFSE)-labeled epididymosomes (n = 4 mice), and visualized using high-resolution confocal microscopy.
Participants/materials, setting, methods: Adult (12-week-old) C57BL/CBAF1 wild-type male mice and adult humans were used for validation purposes. Testes and epididymides from both mice and humans were obtained and processed for immunofluorescence. Mouse epididymal sperm and mouse epididymosomes were obtained from the epididymal cauda segment. Fluorescent epididymosomes were obtained after labeling the epididymal vesicles with CFSE dye followed by epididymosome isolation using a density cushion. Immunofluorescence was performed following co-incubation of sperm with epididymosomes in vitro. High-resolution confocal microscopy and 3D image reconstruction were used to visualize protein localization and sperm-epididymosomes interactions.
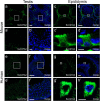
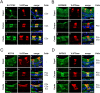
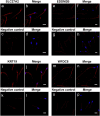
Main results and the role of chance: Through in silico analysis, we first identified 25 sperm proteins with a putative epididymal origin that were conserved in both human and mouse spermatozoa. From those, the epididymal origin of four sperm proteins (SLC27A2, EDDM3B, KRT19 and WFDC8) was validated by high-resolution confocal microscopy. SLC27A2, EDDM3B, KRT19 and WFDC8 were all detected in epithelial cells lining the human and mouse epididymis, and absent from human and mouse seminiferous tubules. We found region-specific expression patterns of these proteins throughout the mouse epididymides. In addition, while EDDM3B, KRT19 and WFDC8 were detected in both epididymal principal and clear cells (CCs), SLC27A2 was exclusively expressed in CCs. Finally, we showed that CFSE-fluorescently labeled epididymosomes interact with sperm in vitro and about 12-36% of the epididymosomes contain the targeted sperm proteins with an epididymal origin.
Large scale data: N/A.
Limitations, reasons for caution: The human and mouse sample size was limited and our results were descriptive. The analyses of epididymal sperm and epididymosomes were solely performed in the mouse model due to the difficulties in obtaining epididymal luminal fluid human samples. Alternatively, human ejaculated sperm and seminal EVs could not be used because ejaculated sperm have already contacted with the fluids secreted by the male accessory sex glands, and seminal EVs contain other EVs in addition to epididymosomes, such as the abundant prostate-derived EVs.
Wider implications of the findings: Our findings indicate that epididymosomes are capable of providing spermatozoa with a new set of epididymis-derived proteins that could modulate the sperm proteome and, subsequently, participate in the post-testicular maturation of sperm cells. Additionally, our data provide further evidence of the novel role of epididymal CCs in epididymosome production. Identifying mechanisms by which sperm mature to acquire their fertilization potential would, ultimately, lead to a better understanding of male reproductive health and may help to identify potential therapeutic strategies to improve male infertility.
Study funding/competing interest(s): This work was supported by the Spanish Ministry of Economy and Competitiveness (Ministerio de Economía y Competividad; fondos FEDER 'una manera de hacer Europa' PI13/00699 and PI16/00346 to R.O.; and Sara Borrell Postdoctoral Fellowship, Acción Estratégica en Salud, CD17/00109 to J.C.), by National Institutes of Health (grants HD040793 and HD069623 to S.B., grant HD104672-01 to M.A.B.), by the Spanish Ministry of Education, Culture and Sports (Ministerio de Educación, Cultura y Deporte para la Formación de Profesorado Universitario, FPU15/02306 to F.B.), by a Lalor Foundation Fellowship (to F.B. and M.A.B.), by the Government of Catalonia (Generalitat de Catalunya, pla estratègic de recerca i innovació en salut, PERIS 2016-2020, SLT002/16/00337 to M.J.), by Fundació Universitària Agustí Pedro i Pons (to F.B.), and by the American Society for Biochemistry and Molecular Biology (PROLAB Award from ASBMB/IUBMB/PABMB to F.B.). Confocal microscopy and transmission electron microscopy was performed in the Microscopy Core facility of the Massachusetts General Hospital (MGH) Center for Systems Biology/Program in Membrane Biology which receives support from Boston Area Diabetes and Endocrinology Research Center (BADERC) award DK57521 and Center for the Study of Inflammatory Bowel Disease grant DK43351. The Zeiss LSM800 microscope was acquired using an NIH Shared Instrumentation Grant S10-OD-021577-01. The authors have no conflicts of interest to declare.
Keywords: epididymis; epididymosomes; exosomes; extracellular vesicles; male fertility; post-testicular maturation; protein transfer; sperm maturation; sperm proteins; sperm proteome.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Histone H4 acetylation is dysregulated in active seminiferous tubules adjacent to testicular tumours.Hum Reprod. 2022 Jul 30;37(8):1712-1726. doi: 10.1093/humrep/deac130. Hum Reprod. 2022. PMID: 35678707
-
Junctional adhesion molecule A: expression in the murine epididymal tract and accessory organs and acquisition by maturing sperm.Mol Hum Reprod. 2017 Feb 10;23(2):132-140. doi: 10.1093/molehr/gaw082. Mol Hum Reprod. 2017. PMID: 28062807 Free PMC article.
-
Role of exosomes in sperm maturation during the transit along the male reproductive tract.Blood Cells Mol Dis. 2005 Jul-Aug;35(1):1-10. doi: 10.1016/j.bcmd.2005.03.005. Blood Cells Mol Dis. 2005. PMID: 15893944 Review.
-
Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation.Mol Cell Proteomics. 2019 Mar 15;18(Suppl 1):S91-S108. doi: 10.1074/mcp.RA118.000946. Epub 2018 Sep 13. Mol Cell Proteomics. 2019. PMID: 30213844 Free PMC article.
-
The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction.Int J Mol Sci. 2020 Jul 29;21(15):5377. doi: 10.3390/ijms21155377. Int J Mol Sci. 2020. PMID: 32751076 Free PMC article. Review.
Cited by
-
Factors to consider before choosing EV labeling method for fluorescence-based techniques.Front Bioeng Biotechnol. 2024 Sep 18;12:1479516. doi: 10.3389/fbioe.2024.1479516. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39359260 Free PMC article. Review.
-
The role of CoQ10 in embryonic development.J Assist Reprod Genet. 2024 Mar;41(3):767-779. doi: 10.1007/s10815-024-03052-6. Epub 2024 Feb 19. J Assist Reprod Genet. 2024. PMID: 38372883 Free PMC article. Review.
-
Immunoregulatory mechanisms between epithelial clear cells and mononuclear phagocytes in the epididymis.Andrology. 2024 Jul;12(5):949-963. doi: 10.1111/andr.13509. Epub 2023 Aug 12. Andrology. 2024. PMID: 37572347 Free PMC article.
-
Stress increases sperm respiration and motility in mice and men.Nat Commun. 2024 Sep 11;15(1):7900. doi: 10.1038/s41467-024-52319-0. Nat Commun. 2024. PMID: 39261485 Free PMC article.
-
Effect of C-Type Natriuretic Peptide (CNP) on Spermatozoa Maturation in Adult Rat Epididymis.Curr Issues Mol Biol. 2023 Feb 16;45(2):1681-1692. doi: 10.3390/cimb45020108. Curr Issues Mol Biol. 2023. PMID: 36826053 Free PMC article.
References
-
- Aalberts M, Stout TAE, Stoorvogel W.. Prostasomes: extracellular vesicles from the prostate. Reproduction 2014;147:R1–R14. - PubMed
-
- Amaral A, Paiva C, Attardo Parrinello C, Estanyol JM, Ballescà JL, Ramalho-Santos J, Oliva R.. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J Proteome Res 2014;13:5670–5684. - PubMed
-
- Baker MA, Hetherington L, Reeves GM, Aitken RJ.. The mouse sperm proteome characterized via IPG strip prefractionation and LC-MS/MS identification. Proteomics 2008;8:1720–1730. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

