Using guideline-based clinical decision support in oncological multidisciplinary team meetings: A prospective, multicenter concordance study
- PMID: 35137091
- PMCID: PMC8934031
- DOI: 10.1093/intqhc/mzac007
Using guideline-based clinical decision support in oncological multidisciplinary team meetings: A prospective, multicenter concordance study
Abstract
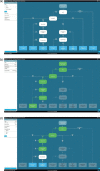
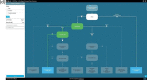
Background: Multidisciplinary team meetings formulate guideline-based individual treatment plans based on patient and disease characteristics and motivate reasons for deviation. Clinical decision trees could support multidisciplinary teams to adhere more accurately to guidelines. Every clinical decision tree is tailored to a specific decision moment in a care pathway and is composed of patient and disease characteristics leading to a guideline recommendation.
Objective: This study investigated (1) the concordance between multidisciplinary team and clinical decision tree recommendations and (2) the completeness of patient and disease characteristics available during multidisciplinary team meetings to apply clinical decision trees such that it results in a guideline recommendation.
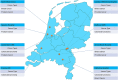
Methods: This prospective, multicenter, observational concordance study evaluated 17 selected clinical decision trees, based on the prevailing Dutch guidelines for breast, colorectal and prostate cancers. In cases with sufficient data, concordance between multidisciplinary team and clinical decision tree recommendations was classified as concordant, conditional concordant (multidisciplinary team specified a prerequisite for the recommendation) and non-concordant.
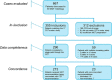
Results: Fifty-nine multidisciplinary team meetings were attended in 8 different hospitals, and 355 cases were included. For 296 cases (83.4%), all patient data were available for providing an unconditional clinical decision tree recommendation. In 59 cases (16.6%), insufficient data were available resulting in provisional clinical decision tree recommendations. From the 296 successfully generated clinical decision tree recommendations, the multidisciplinary team recommendations were concordant in 249 (84.1%) cases, conditional concordant in 24 (8.1%) cases and non-concordant in 23 (7.8%) cases of which in 7 (2.4%) cases the reason for deviation from the clinical decision tree generated guideline recommendation was not motivated.
Conclusion: The observed concordance of recommendations between multidisciplinary teams and clinical decision trees and data completeness during multidisciplinary team meetings in this study indicate a potential role for implementation of clinical decision trees to support multidisciplinary team decision-making.
Keywords: algorithms; clinical decision support system; clinical decision trees; clinical practice guidelines; multidisciplinary team meeting; oncology.
© The Author(s) 2022. Published by Oxford University Press on behalf of International Society for Quality in Health Care.
Figures
References
-
- Elsevier . Cancer research current trends & future directions. 2016.
-
- Metcalfe D, Pitkeathley C, Herring J. ‘Advice, not orders’? The evolving legal status of clinical guidelines. J Med Ethics Medethics 2020;47:e78. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

