A random survey of the prevalence of falsified and substandard antibiotics in the Lao PDR
- PMID: 35137095
- PMCID: PMC7614350
- DOI: 10.1093/jac/dkab435
A random survey of the prevalence of falsified and substandard antibiotics in the Lao PDR
Erratum in
-
Editor's note.J Antimicrob Chemother. 2022 May 29;77(6):1791. doi: 10.1093/jac/dkab458. J Antimicrob Chemother. 2022. PMID: 35137082 Free PMC article. No abstract available.
Abstract
Objectives: In 2012, a stratified random survey, using mystery shoppers, was conducted to investigate the availability and quality of antibiotics sold to patients in the private sector in five southern provinces of the Lao People's Democratic Republic (Laos).
Methods: A total of 147 outlets were sampled in 10 districts. The active pharmaceutical ingredient (API) content measurements for 909 samples, including nine APIs (amoxicillin, ampicillin, ceftriaxone, ciprofloxacin, doxycycline, ofloxacin, sulfamethoxazole, tetracycline and trimethoprim), were determined using HPLC.
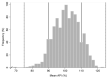
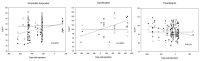
Results: All the analysed samples contained the stated API and we found no evidence for falsification. All except one sample had all the units tested with %API values between 75% and 125% of the content stated on the label. However, we identified the presence of substandard antibiotics: 19.6% (201/1025) of samples had their units outside the 90%-110% content of the label claim and 18.3% (188/1025) of the samples had units outside the International Pharmacopoeia/United States Pharmacopoeia assay (percentage of label claim) specifications. Trimethoprim had a high number of samples [51.6% (64)] with units below the limit range, followed by ceftriaxone [42.9% (3)] and sulfamethoxazole [34.7% (43)]. Doxycycline, ofloxacin and ciprofloxacin had the highest number of samples with high API content: 43.7% (38), 14.7% (10) and 11.8% (2), respectively. Significant differences in %API were found between stated countries of manufacture and stated manufacturers.
Conclusions: With the global threat of antimicrobial resistance on patient outcomes, greater understanding of the role of poor-quality antibiotics is needed. Substandard antibiotics will have reduced therapeutic efficacy, impacting public health and control of bacterial infections.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures


Similar articles
-
A random survey of the prevalence of falsified and substandard antibiotics in the Lao PDR.J Antimicrob Chemother. 2019 Aug 1;74(8):2417-2425. doi: 10.1093/jac/dkz164. J Antimicrob Chemother. 2019. PMID: 31049576 Free PMC article.
-
A Repeat Random Survey of the Prevalence of Falsified and Substandard Antimalarials in the Lao PDR: A Change for the Better.Am J Trop Med Hyg. 2015 Jun;92(6 Suppl):95-104. doi: 10.4269/ajtmh.15-0057. Epub 2015 Apr 20. Am J Trop Med Hyg. 2015. PMID: 25897062 Free PMC article.
-
Substandard and Falsified Antibiotics and Medicines against Noncommunicable Diseases in Western Cameroon and Northeastern Democratic Republic of Congo.Am J Trop Med Hyg. 2020 Aug;103(2):894-908. doi: 10.4269/ajtmh.20-0184. Epub 2020 May 7. Am J Trop Med Hyg. 2020. PMID: 32394884 Free PMC article.
-
Characterizing Medicine Quality by Active Pharmaceutical Ingredient Levels: A Systematic Review and Meta-Analysis across Low- and Middle-Income Countries.Am J Trop Med Hyg. 2022 Jun 15;106(6):1778-1790. doi: 10.4269/ajtmh.21-1123. Print 2022 Jun 15. Am J Trop Med Hyg. 2022. PMID: 35895431 Free PMC article.
-
Private sector opportunities and threats to achieving malaria elimination in the Greater Mekong Subregion: results from malaria outlet surveys in Cambodia, the Lao PDR, Myanmar, and Thailand.Malar J. 2017 May 2;16(1):180. doi: 10.1186/s12936-017-1800-5. Malar J. 2017. PMID: 28464945 Free PMC article. Review.
Cited by
-
Estimating the prevalence of poor-quality anti-TB medicines: a neglected risk for global TB control and resistance.BMJ Glob Health. 2023 Jul;8(7):e012039. doi: 10.1136/bmjgh-2023-012039. BMJ Glob Health. 2023. PMID: 37433693 Free PMC article.
-
Editor's note.J Antimicrob Chemother. 2022 May 29;77(6):1791. doi: 10.1093/jac/dkab458. J Antimicrob Chemother. 2022. PMID: 35137082 Free PMC article. No abstract available.
-
Assessing the quality of amoxicillin in the private market in Indonesia: a cross-sectional survey exploring product variety, market volume and price factors.BMJ Open. 2025 Jul 22;15(7):e093785. doi: 10.1136/bmjopen-2024-093785. BMJ Open. 2025. PMID: 40701591 Free PMC article.
-
Extensively and multidrug-resistant bacterial strains: case studies of antibiotics resistance.Front Microbiol. 2024 Jul 4;15:1381511. doi: 10.3389/fmicb.2024.1381511. eCollection 2024. Front Microbiol. 2024. PMID: 39027098 Free PMC article. Review.
-
A comparative field evaluation of six medicine quality screening devices in Laos.PLoS Negl Trop Dis. 2021 Sep 30;15(9):e0009674. doi: 10.1371/journal.pntd.0009674. eCollection 2021 Sep. PLoS Negl Trop Dis. 2021. PMID: 34591852 Free PMC article.
References
-
- Odermatt P, Ly S, Simmala C, et al. Availability and costs of antiepileptic drugs and quality of phenobarbital in Vientiane municipality, Lao PDR. Neuroepidemiology. 2007;28:169–74. - PubMed
-
- WHO. Antimicrobial Resistance Factsheet. http://www.who.int/mediacentre/factsheets/fs194/en/
-
- WHO. The Evolving Threat of Antimicrobial Resistance: Options for Action. http://apps.who.int/iris/bitstream/10665/44812/1/9789241503181_eng.pdf .
-
- WHO. WHO Global Strategy for Containment of Antimicrobial Resistance. http://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf .
-
- Sosa AJ. Antimicrobial Resistance in Developing Countries. New York: Springer; 2010. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

