Pramlintide for post-bariatric hypoglycaemia
- PMID: 35137513
- PMCID: PMC9035096
- DOI: 10.1111/dom.14665
Pramlintide for post-bariatric hypoglycaemia
Abstract
Aims: The aim of this study was to examine the hypothesis that pramlintide would reduce hypoglycaemia by slowing gastric emptying and reducing postprandial glucagon secretion, thus limiting postprandial glycaemic excursions and insulin secretion, and thus to determine the efficacy of pramlintide on frequency and severity of hypoglycaemia in post-bariatric hypoglycaemia (PBH).
Materials and methods: Participants with PBH following gastric bypass were recruited from outpatient clinics at the Joslin Diabetes Center, Boston, Massachusetts for an open-label study of pramlintide efficacy over 8 weeks. Twenty-three participants were assessed for eligibility, 20 participants had at least one pramlintide dose, and 14 completed the study. A mixed-meal tolerance test (MMTT) was performed at baseline and after 8 weeks of subcutaneous pramlintide with a sequential dose increase to a maximum of 120 micrograms (mean 69 ± 32 mcg) three times daily. The primary endpoint was change in glucose excursions during the MMTT. Secondary measures included MMTT insulin response, satiety and dumping score, percentage time with sensor glucose (SG) <3.9 mM, and number of days with minimum SG <3 mM, during masked continuous glucose monitoring.
Results: There were no differences in MMTT glucose, glucagon or insulin between baseline and post treatment. We observed no significant change in satiety or dumping scores. The overall frequency of low SG values did not change, although there was substantial inter-individual variability.
Conclusions: In PBH, pramlintide does not modulate glycaemic or insulin responses, satiety, or dumping scores during an MMTT and does not impact glycaemic excursions or decrease low SG levels in the outpatient setting.
Keywords: bariatric surgery; continuous glucose monitoring (CGM); glucagon; hypoglycaemia.
© 2022 John Wiley & Sons Ltd.
Figures
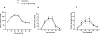
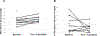

References
-
- Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet (London, England). 2021;397(10271). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

