What Should be the First-line Treatment for the Closure of Hemodynamically Significant Patent Ductus Arteriosus in Premature Infants?
- PMID: 35137787
- PMCID: PMC8959034
- DOI: 10.36660/abc.20201361
What Should be the First-line Treatment for the Closure of Hemodynamically Significant Patent Ductus Arteriosus in Premature Infants?
Abstract
Background: It is important which medicine to use as a first-line treatment to close the duct.
Objectives: The aim of this study is to compare the effectiveness and side effects of intravenous (IV) forms of ibuprofen and paracetamol and to contribute to the literature investigating the first drug selected in the medical treatment of patent ductus arteriosus (PDA).
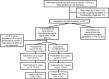
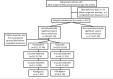
Methods: Our study was conducted between January 2017 and December 2019. Premature infants with birth weight (BW) ≤1500 g and gestational age (GA) ≤32 weeks were included in the study. In the study period, all infants with hemodynamically significant patent ductus arteriosus (hsPDA) were given rescue intravenous (IV) ibuprofen as a primary medical treatment or IV paracetamol treatment if there were contraindications for ibuprofen. The patients were divided into two groups: patients receiving IV ibuprofen and patients receiving IV paracetamol.
Results: Of these patients, 101 were given IV paracetamol and 169 were given IV ibuprofen. The success rate of PDA closure with first-course treatment was 74.3% in the IV paracetamol group and 72.8% in the IV ibuprofen group (p=0.212).
Conclusions: Our results show that IV paracetamol is as effective as IV ibuprofen in the first-line treatment of hsPDA, and can become the preferred treatment for the management of hsPDA.
Fundamento: É importante saber qual medicamento usar como tratamento de primeira linha para fechar o duto.
Objetivos: O objetivo deste estudo é comparar a eficácia e os efeitos colaterais das formas intravenosas (IV) de ibuprofeno e paracetamol e contribuir para a literatura investigando o primeiro medicamento selecionado no tratamento clínico da persistência do canal arterial (PCA).
Métodos: Nosso estudo foi realizado entre janeiro de 2017 e dezembro de 2019. Foram incluídos no estudo bebês prematuros com peso ao nascer (PN) ≤1500 g e idade gestacional (IG) ≤32 semanas. No período do estudo, todos os bebês com persistência do canal arterial hemodinamicamente significativa (hsPCA) receberam ibuprofeno intravenoso (IV) como resgate como tratamento clínico primário ou tratamento com paracetamol IV se houvesse contraindicações para o ibuprofeno. Os pacientes foram divididos em dois grupos: pacientes que receberam ibuprofeno IV e pacientes que receberam paracetamol IV.
Resultados: Desses pacientes, 101 receberam paracetamol IV e 169 receberam ibuprofeno IV. A taxa de sucesso do fechamento da PCA com o primeiro curso do tratamento foi de 74,3% no grupo de paracetamol IV e 72,8% no grupo de ibuprofeno IV (p=0,212).
Conclusões: Nossos resultados mostram que o paracetamol IV é tão eficaz quanto o ibuprofeno IV no tratamento de primeira linha de hsPCA, podendo se tornar o tratamento preferencial para o controle de hsPCA.
Conflict of interest statement
Potencial conflito de interesse
Não há conflito com o presente artigo
Figures
Similar articles
-
Comparison of the effect of continuous and standard intermittent bolus paracetamol infusion on patent ductus arteriosus.Eur J Pediatr. 2021 Feb;180(2):433-440. doi: 10.1007/s00431-020-03822-1. Epub 2020 Sep 29. Eur J Pediatr. 2021. PMID: 32995919
-
Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates.Eur J Pediatr. 2017 Feb;176(2):233-240. doi: 10.1007/s00431-016-2830-7. Epub 2016 Dec 21. Eur J Pediatr. 2017. PMID: 28004188 Clinical Trial.
-
Efficacy and safety of oral paracetamol versus oral ibuprofen for closure of patent ductus arteriosus in preterm infants: a randomized controlled trial.J Matern Fetal Neonatal Med. 2019 Nov;32(21):3647-3654. doi: 10.1080/14767058.2018.1470235. Epub 2018 May 9. J Matern Fetal Neonatal Med. 2019. PMID: 29695206 Clinical Trial.
-
Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants.Cochrane Database Syst Rev. 2020 Feb 11;2(2):CD003481. doi: 10.1002/14651858.CD003481.pub8. Cochrane Database Syst Rev. 2020. PMID: 32045960 Free PMC article.
-
Therapeutic Efficacy and Safety of Paracetamol versus Ibuprofen in Patent Ductus Arteriosus in Newborns: A Systematic Review.Arq Bras Cardiol. 2024 Dec 6;121(11):e20240058. doi: 10.36660/abc.20240058. eCollection 2024. Arq Bras Cardiol. 2024. PMID: 39661802 Free PMC article. English, Portuguese.
References
-
- Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164(3):510-4.e1. - PubMed
- Oncel MY, Yurttutan S, Erdeve O, Uras N, Altug N, Oguz SS, et al. Oral paracetamol versus oral ibuprofen in the management of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Pediatr. 2014;164(3):510–4.e1. - PubMed
-
- Karabulut B, Paytoncu S. Efficacy and Safety of Oral Paracetamol vs. Oral Ibuprofen in the Treatment of Symptomatic Patent Ductus Arteriosus in Premature Infants. Paediatr Drugs. 2019;21(2):113-21. - PubMed
- Karabulut B, Paytoncu S. Efficacy and Safety of Oral Paracetamol vs. Oral Ibuprofen in the Treatment of Symptomatic Patent Ductus Arteriosus in Premature Infants. Paediatr Drugs. 2019;21(2):113–121. - PubMed
-
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344(26):1966-72. - PubMed
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344(26):1966–1972. - PubMed
-
- Dani C, Bertini G, Pezzati M, Poggi C, Guerrini P, Martano C, et al. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics. 2005;115(6):1529-35. - PubMed
- Dani C, Bertini G, Pezzati M, Poggi C, Guerrini P, Martano C, et al. Prophylactic ibuprofen for the prevention of intraventricular hemorrhage among preterm infants: a multicenter, randomized study. Pediatrics. 2005;115(6):1529–1535. - PubMed
-
- Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160(6):929-35.e1. - PubMed
- Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160(6):929–35.e1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources