Use of remdesivir in patients with COVID-19: a systematic review and meta-analysis
- PMID: 35137874
- PMCID: PMC8836613
- DOI: 10.36416/1806-3756/e20210393
Use of remdesivir in patients with COVID-19: a systematic review and meta-analysis
Abstract
Objective: Studies in the literature regarding the use of remdesivir to treat COVID-19 patients have shown conflicting results. This study sought to answer questions related to the use of remdesivir for the treatment of patients hospitalized with moderate to severe COVID-19.
Methods: This was a systematic review and meta-analysis including phase 3 randomized clinical trials (RCTs) and observational cohort studies selected from various databases, comparing patients hospitalized with moderate to severe COVID-19 receiving remdesivir and controls.
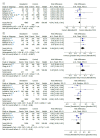
Results: A total of 207 studies were retrieved, 9 of which met the eligibility criteria and were included in the study. The meta-analysis using RCTs alone showed no statistically significant differences regarding mortality or use of mechanical ventilation/extracorporeal membrane oxygenation between remdesivir and control groups, and the quality of evidence was moderate and low, respectively. The use of remdesivir increased the recovery rate by 6% (95% CI, 3-9); p = 0.004) and the clinical improvement rate by 7% (95% CI, 1-14); p = 0.02). Additionally, no significant differences in mortality were found between remdesivir and control groups when the meta-analysis used observational cohort studies alone (risk difference = -0.01 (95% CI, -0.02 to 0.01; p = 0.32), the quality of evidence being moderate, and the risk of adverse events was 4% ([95% CI, -0.08 to 0.01]; p = 0.09).
Conclusions: The use of remdesivir for the treatment of patients with moderate to severe COVID-19 had no significant impact on clinically important outcomes.
Objetivo:: Estudos na literatura sobre o uso de remdesivir no tratamento de pacientes com COVID-19 têm apresentado resultados divergentes. O objetivo deste estudo foi responder a perguntas a respeito do uso de remdesivir no tratamento de pacientes hospitalizados com COVID-19 moderada a grave.
Métodos:: Trata-se de uma revisão sistemática e meta-análise de ensaios clínicos controlados randomizados (ECR) de fase 3 e estudos observacionais de coorte recuperados de diversos bancos de dados, comparando pacientes hospitalizados com COVID-19 moderada a grave recebendo remdesivir a controles.
Resultados:: Foram recuperados 207 estudos, dos quais 9 preencheram os critérios de elegibilidade e foram incluídos no estudo. A meta-análise somente dos ECR não mostrou diferenças estatisticamente significativas entre os grupos remdesivir e controle quanto à mortalidade ou ao uso de ventilação mecânica/oxigenação por membrana extracorpórea, e a qualidade das evidências foi moderada e baixa, respectivamente. O uso de remdesivir aumentou a taxa de recuperação em 6% (IC95%: 3-9; p = 0,004) e a taxa de melhora clínica em 7% (IC95%: 1-14; p = 0,02). Além disso, não foram observadas diferenças significativas entre os grupos remdesivir e controle quanto à mortalidade quando a meta-análise concentrou-se apenas nos estudos observacionais de coorte [diferença de risco = −0,01 (IC95%: −0,02 a 0,01); p = 0,32; qualidade das evidências: moderada], e o risco de eventos adversos foi de 4% (IC95%: −0,08 a 0,01; p = 0,09).
Conclusões:: O uso de remdesivir no tratamento de pacientes com COVID-19 moderada a grave não teve impacto significativo em desfechos clinicamente importantes.
Conflict of interest statement
Figures


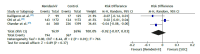
References
-
- World Health Organization . WHO Coronavirus (COVID-19) Dashboard. Geneva: WHO; 2021. https://covid19.who.int
-
- World Health Organization . Brazil: WHO Coronavirus (COVID-19) Dashboard. Geneva: WHO; c2021. https://covid19.who.int/region/amro/country/br
-
- Gerard L, Lecocq M, Bouzin C, Hoton D, Schmit G, Pereira JP, et al. ncreased Angiotensin-Converting Enzyme 2 and Loss of Alveolar Type II Cells in COVID-19-related Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2021;204(9):1024–1034. doi: 10.1164/rccm.202012-4461OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

