Biomarker Qualification at the European Medicines Agency: A Review of Biomarker Qualification Procedures From 2008 to 2020
- PMID: 35137949
- PMCID: PMC9313861
- DOI: 10.1002/cpt.2554
Biomarker Qualification at the European Medicines Agency: A Review of Biomarker Qualification Procedures From 2008 to 2020
Abstract
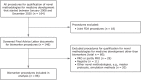
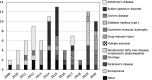
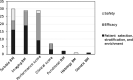
Regulatory qualification of biomarkers facilitates their harmonized use across drug developers, enabling more personalized medicine. This study reviews various aspects of the European Medicines Agency's (EMA's) biomarker qualification procedure, including frequency and outcome, common challenges, and biomarker characteristics. Our findings provide insights into the EMA's biomarker qualification process and will thereby support future applications. All biomarker-related "Qualification of Novel Methodologies for Medicine Development" procedures that started from 2008 to 2020 were included. Procedural data were extracted from relevant documents and analyzed descriptively. In total, 86 biomarker qualification procedures were identified, of which 13 resulted in qualified biomarkers. Whereas initially many biomarker qualification procedures were linked to a single company and specific drug development program, a shift was observed to qualification efforts by consortia. Most biomarkers were proposed (n = 45) and qualified (n = 9) for use in patient selection, stratification, and/or enrichment, followed by efficacy biomarkers (37 proposed, 4 qualified). Overall, many issues were raised during qualification procedures, mostly related to biomarker properties and assay validation (in 79% and 77% of all procedures, respectively). Issues related to the proposed context of use and rationale were least common yet were still raised in 54% of all procedures. While few qualified biomarkers are currently available, procedures focus increasingly on biomarkers for general use instead of those linked to specific drug compounds. The issues raised during qualification procedures illustrate the thorough discussions taking place between applicants and regulators-highlighting aspects that need careful consideration and underlining the importance of an appropriate validation strategy.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
E.B. received funding from the Biomarker Enterprise to Attack DKD (BEAT‐DKD). All other authors declared no competing interests for this work.
Figures

Comment in
-
Biomarker Qualification at the European Medicines Agency: A Look Under the Hood.Clin Pharmacol Ther. 2022 Jul;112(1):28-30. doi: 10.1002/cpt.2609. Epub 2022 May 2. Clin Pharmacol Ther. 2022. PMID: 35500535 Free PMC article. No abstract available.
References
-
- Amur, S. , LaVange, L. , Zineh, I. , Buckman‐Garner, S. & Woodcock, J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin. Pharmacol. Ther. 98, 34–46 (2015). - PubMed
-
- FDA‐NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet] (Food and Drug Administration (US) and National Institutes of Health (US), Bethesda (MD), Silver Spring, MD, 2016). <https://www.ncbi.nlm.nih.gov/books/NBK326791/>. Accessed February 23, 2022. - PubMed
-
- Galante, D. & Buyse, M. Integrating personalized medicine in the health care. In Handbook of Biomarkers and Precision Medicine 1st edition (eds. Carini, C. et al.) (Chapman and Hall/CRC, Boca Raton, 2019). < https://www.taylorfrancis.com/chapters/edit/10.1201/9780429202872‐3/inte...>. Accessed February 23, 2022. - DOI
-
- Lathia, C.D. et al. The value, qualification, and regulatory use of surrogate end points in drug development. Clin. Pharmacol. Ther. 86, 32–43 (2009). - PubMed
-
- Landeck, L. , Kneip, C. , Reischl, J. & Asadullah, K. Biomarkers and personalized medicine: current status and further perspectives with special focus on dermatology. Exp. Dermatol. 25, 333–339 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

