Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors
- PMID: 35138042
- PMCID: PMC8981489
- DOI: 10.1002/advs.202105152
Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors
Abstract
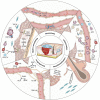
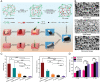
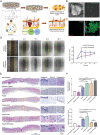
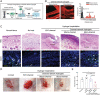
Skin wound repair is a multistage process involving multiple cellular and molecular interactions, which modulate the cell behaviors and dynamic remodeling of extracellular matrices to maximize regeneration and repair. Consequently, abnormalities in cell functions or pathways inevitably give rise to side effects, such as dysregulated inflammation, hyperplasia of nonmigratory epithelial cells, and lack of response to growth factors, which impedes angiogenesis and fibrosis. These issues may cause delayed wound healing or even non-healing states. Current clinical therapeutic approaches are predominantly dedicated to preventing infections and alleviating topical symptoms rather than addressing the modulation of wound microenvironments to achieve targeted outcomes. Bioactive materials, relying on their chemical, physical, and biological properties or as carriers of bioactive substances, can affect wound microenvironments and promote wound healing at the molecular level. By addressing the mechanisms of wound healing from the perspective of cell behaviors, this review discusses how bioactive materials modulate the microenvironments and cell behaviors within the wounds during the stages of hemostasis, anti-inflammation, tissue regeneration and deposition, and matrix remodeling. A deeper understanding of cell behaviors during wound healing is bound to promote the development of more targeted and efficient bioactive materials for clinical applications.
Keywords: bioactive material; cell behavior; regenerative medicine; wound healing; wound microenvironment.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mathes S. H., Ruffner H., Graf‐Hausner U., Adv. Drug Deliv. Rev. 2014, 69, 81. - PubMed
-
- Kim H. S., Sun X., Lee J. H., Kim H. W., Fu X., Leong K. W., Adv. Drug Deliv. Rev. 2019, 146, 209. - PubMed
-
- Castano O., Perez‐Amodio S., Navarro‐Requena C., Mateos‐Timoneda M. A., Engel E., Adv. Drug Deliv. Rev. 2018, 129, 95. - PubMed
Publication types
MeSH terms
Grants and funding
- 52022095/National Natural Science Foundation of China
- 82071391/National Natural Science Foundation of China
- 51973216/National Natural Science Foundation of China
- 51873207/National Natural Science Foundation of China
- 51833010/National Natural Science Foundation of China
- JLSWSRCZX2020-095/Provincial Health Special Project of Jilin Province
- JLSCZD2019-002/Provincial Health Special Project of Jilin Province
- 20210509005RQ/Science and Technology Development Program of Jilin Province
- 20200404182YY/Science and Technology Development Program of Jilin Province
- 2019230/Youth Innovation Promotion Association of Chinese Academy of Sciences
- 20200404182YY/Department of Science and Technology of Jilin Province
- 20210509005RQ/Program for Jilin University Science and Technology Innovative Research Team
LinkOut - more resources
Full Text Sources
