The Zinc Finger Transcription Factor BbCmr1 Regulates Conidium Maturation in Beauveria bassiana
- PMID: 35138172
- PMCID: PMC8826823
- DOI: 10.1128/spectrum.02066-21
The Zinc Finger Transcription Factor BbCmr1 Regulates Conidium Maturation in Beauveria bassiana
Abstract
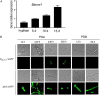
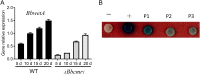
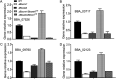
The entomopathogenic fungus Beauveria bassiana is a typical filamentous fungus and has been used for pest biocontrol. Conidia are the main active agents of fungal pesticides; however, we know little about conidial developmental mechanisms and less about maturation mechanisms. We found that a Zn2Cys6 transcription factor of B. bassiana (named BbCmr1) was mainly expressed in late-stage conidia and was involved in conidium maturation regulation. Deletion of Bbcmr1 impaired the conidial cell wall and resulted in a lower conidial germination rate under UV (UV), heat shock, H2O2, Congo red (CR) and SDS stresses compared to the wild type. Transcription levels of the genes associated with conidial wall components and trehalose synthase were significantly reduced in the ΔBbcmr1 mutant. Further analysis found that BbCmr1 functions by upregulating BbWetA, a well-known transcription factor in the central development of BrlA-AbaA-WetA. The expression of Bbcmr1 was positively regulated by BbBrlA. These results indicated that BbCmr1 played important roles in conidium maturation by interacting with the central development pathway, which provided insight into the conidial development networks in B. bassiana. IMPORTANCE Conidium maturation is a pivotal event in conidial development and affects fungal survival ability under various biotic/abiotic stresses. Although many transcription factors have been reported to regulate conidial development, we know little about the molecular mechanism of conidium maturation. Here, we demonstrated that the transcription factor BbCmr1 of B. bassiana was involved in conidium maturation, regulating cell wall structure, the expression of cell wall-related proteins, and trehalose synthesis. BbCmr1 orchestrated conidium maturation by interplaying with the central development pathway BrlA-AbaA-WetA. BbBrlA positively regulated the expression of Bbcmr1, and the latter positively regulated BbwetA expression, which forms a regulatory network mediating conidial development. This finding was critical to understand the molecular regulatory networks of conidial development in B. bassiana and provided avenues to engineer insect fungal pathogens with high-quality conidia.
Keywords: BbCmr1; BbWetA; Beauveria bassiana; conidium maturation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

