Irx3 promotes gap junction communication between uterine stromal cells to regulate vascularization during embryo implantation†
- PMID: 35138358
- PMCID: PMC9113487
- DOI: 10.1093/biolre/ioac015
Irx3 promotes gap junction communication between uterine stromal cells to regulate vascularization during embryo implantation†
Abstract
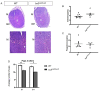
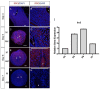
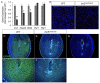
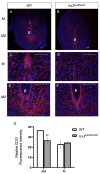
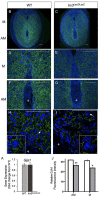
Appropriate embryo-uterine interactions are essential for implantation. Besides oocyte abnormalities, implantation failure is a major contributor to early pregnancy loss. Previously, we demonstrated that two members of the Iroquois homeobox transcription factor family, IRX3 and IRX5, exhibited distinct and dynamic expression profiles in the developing ovary to promote oocyte and follicle survival. Elimination of each gene independently caused subfertility, but with different breeding pattern outcomes. Irx3 KO (Irx3LacZ/LacZ) females produced fewer pups throughout their reproductive lifespan which could only be partially explained by poor oocyte quality. Thus, we hypothesized that IRX3 is also expressed in the uterus where it acts to support pregnancy. To test this hypothesis, we harvested pregnant uteri from control and Irx3 KO females to evaluate IRX3 expression profiles and the integrity of embryo implantation sites. Our results indicate that IRX3 is expressed in the endometrial stromal cells at day 4 of pregnancy (D4) with peak expression at D5-D6, and then greatly diminishes by D7. Further, studies showed that while embryos were able to attach to the uterus, implantation sites in Irx3 KO pregnant mice exhibited impaired vascularization and abnormal expression of decidualization markers. Finally, we also observed an impaired response of the Irx3 KO uteri to an artificial deciduogenic stimulus, indicating a critical role of this factor in regulating the decidualization program. Together, these data established that IRX3 promotes female fertility via at least two different mechanisms: (1) promoting competent oocytes and (2) facilitating functional embryo-uterine interactions during implantation.
Keywords: Angiogenesis; Decidualization; Fertility; Iroquois; Pregnancy; Uterus.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Psychoyos A. Implantation. In: Greep RO, Astwood EG, Geiger SR (eds.), Handbook of Physiology. Washington DC: American Physiology Society; 1973: 187–215.
-
- Yoshinaga K. Uterine receptivity for blastocyst implantation. Ann N Y Acad Sci 1988; 541:424–431. - PubMed
-
- Parr MB, Parr EL. The implantation reaction. In: Wynn RM (ed.), Biology of the Uterus. New York: Plenum Press; 1989: 233–277.
-
- Weitlauf HM. Biology of Implantation. In: Knobil E, Neill JD (eds.), The Physiology of Reproduction. New York: Raven Press Ltd.; 1994: 391–440.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

