Glial Chloride Channels in the Function of the Nervous System Across Species
- PMID: 35138616
- PMCID: PMC11247392
- DOI: 10.1007/978-981-16-4254-8_10
Glial Chloride Channels in the Function of the Nervous System Across Species
Abstract
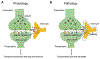
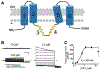
In the nervous system, the concentration of Cl- in neurons that express GABA receptors plays a key role in establishing whether these neurons are excitatory, mostly during early development, or inhibitory. Thus, much attention has been dedicated to understanding how neurons regulate their intracellular Cl- concentration. However, regulation of the extracellular Cl- concentration by other cells of the nervous system, including glia and microglia, is as important because it ultimately affects the Cl- equilibrium potential across the neuronal plasma membrane. Moreover, Cl- ions are transported in and out of the cell, via either passive or active transporter systems, as counter ions for K+ whose concentration in the extracellular environment of the nervous system is tightly regulated because it directly affects neuronal excitability. In this book chapter, we report on the Cl- channel types expressed in the various types of glial cells focusing on the role they play in the function of the nervous system in health and disease. Furthermore, we describe the types of stimuli that these channels are activated by, the other solutes that they may transport, and the involvement of these channels in processes such as pH regulation and Regulatory Volume Decrease (RVD). The picture that emerges is one of the glial cells expressing a variety of Cl- channels, encoded by members of different gene families, involved both in short- and long-term regulation of the nervous system function. Finally, we report data on invertebrate model organisms, such as C. elegans and Drosophila, that are revealing important and previously unsuspected functions of some of these channels in the context of living and behaving animals.
Keywords: Bestrophins; Channelopathies; ClC-2; Glial chloride channels; LRRC8; Maxi chloride channels; Nervous system development; Neuron; Pannexins; SWELL1; VRAC; glia interaction.
© 2021. Springer Nature Singapore Pte Ltd.
Figures






Similar articles
-
A Novel Mechanism of pH Buffering in C. elegans Glia: Bicarbonate Transport via the Voltage-Gated ClC Cl- Channel CLH-1.J Neurosci. 2015 Dec 16;35(50):16377-97. doi: 10.1523/JNEUROSCI.3237-15.2015. J Neurosci. 2015. PMID: 26674864 Free PMC article.
-
A glial ClC Cl- channel mediates nose touch responses in C. elegans.Neuron. 2022 Feb 2;110(3):470-485.e7. doi: 10.1016/j.neuron.2021.11.010. Epub 2021 Dec 2. Neuron. 2022. PMID: 34861150 Free PMC article.
-
Glutamate transporter-associated anion channels adjust intracellular chloride concentrations during glial maturation.Glia. 2017 Feb;65(2):388-400. doi: 10.1002/glia.23098. Epub 2016 Nov 18. Glia. 2017. PMID: 27859594
-
Astrocytic chloride regulates brain function in health and disease.Cell Calcium. 2024 Mar;118:102855. doi: 10.1016/j.ceca.2024.102855. Epub 2024 Feb 7. Cell Calcium. 2024. PMID: 38364706 Review.
-
The signaling role for chloride in the bidirectional communication between neurons and astrocytes.Neurosci Lett. 2019 Jan 10;689:33-44. doi: 10.1016/j.neulet.2018.01.012. Epub 2018 Jan 9. Neurosci Lett. 2019. PMID: 29329909 Free PMC article. Review.
Cited by
-
Role of voltage-gated chloride channels in epilepsy: current insights and future directions.Front Pharmacol. 2025 Mar 28;16:1560392. doi: 10.3389/fphar.2025.1560392. eCollection 2025. Front Pharmacol. 2025. PMID: 40223930 Free PMC article. Review.
-
Physiological roles of chloride ions in bodily and cellular functions.J Physiol Sci. 2023 Nov 15;73(1):31. doi: 10.1186/s12576-023-00889-x. J Physiol Sci. 2023. PMID: 37968609 Free PMC article. Review.
References
-
- Thiemann A, Grunder S, Pusch M, Jentsch TJ (1992) A chloride channel widely expressed in epithelial and non-epithelial cells. Nature 356:57–60 - PubMed
-
- Sirisi S, Elorza-Vidal X, Arnedo T, Armand-Ugon M, Callejo G, Capdevila-Nortes X, Lopez-Hernandez T, Schulte U, Barrallo-Gimeno A, Nunes V, Gasull X, Estevez R (2017) Depolarization causes the formation of a ternary complex between GlialCAM, MLC1 and ClC-2 in astrocytes: implications in megalencephalic leukoencephalopathy. Hum Mol Genet 26:2436–2450 - PubMed
-
- Walz W (2002) Chloride/anion channels in glial cell membranes. Glia 40:1–10 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

