Oral Antihypertensives for Nonsevere Pregnancy Hypertension: Systematic Review, Network Meta- and Trial Sequential Analyses
- PMID: 35138877
- PMCID: PMC8823910
- DOI: 10.1161/HYPERTENSIONAHA.121.18415
Oral Antihypertensives for Nonsevere Pregnancy Hypertension: Systematic Review, Network Meta- and Trial Sequential Analyses
Abstract
Background: We aimed to address which antihypertensives are superior to placebo/no therapy or another antihypertensive for controlling nonsevere pregnancy hypertension and provide future sample size estimates for definitive evidence.
Methods: Randomized trials of antihypertensives for nonsevere pregnancy hypertension were identified from online electronic databases, to February 28, 2021 (registration URL: https://www.crd.york.ac.uk/PROSPERO/; unique identifier: CRD42020188725). Our outcomes were severe hypertension, proteinuria/preeclampsia, fetal/newborn death, small-for-gestational age infants, preterm birth, and admission to neonatal care. A Bayesian random-effects model generated estimates of direct and indirect treatment comparisons. Trial sequential analysis informed future trials needed.
Results: Of 1246 publications identified, 72 trials were included; 61 (6923 women) were informative. All commonly prescribed antihypertensives (labetalol, other β-blockers, methyldopa, calcium channel blockers, and mixed/multi-drug therapy) versus placebo/no therapy reduced the risk of severe hypertension by 30% to 70%. Labetalol decreased proteinuria/preeclampsia (odds ratio, 0.73 [95% credible interval, 0.54-0.99]) and fetal/newborn death (odds ratio, 0.54 [0.30-0.98]) compared with placebo/no therapy, and proteinuria/preeclampsia compared with methyldopa (odds ratio, 0.66 [0.44-0.99]) and calcium channel blockers (odds ratio, 0.63 [0.41-0.96]). No other differences were identified, but credible intervals were wide. Trial sequential analysis indicated that 2500 to 10 000 women/arm (severe hypertension or safety outcomes) to >15 000/arm (fetal/newborn death) would be required to provide definitive evidence.
Conclusions: In summary, all commonly prescribed antihypertensives in pregnancy reduce the risk of severe hypertension, but labetalol may also decrease proteinuria/preeclampsia and fetal/newborn death. Evidence is lacking for many other safety outcomes. Prohibitive sample sizes are required for definitive evidence. Real-world data are needed to individualize care.
Keywords: blood pressure; morbidity; network meta-analysis; proteinuria; sample size.
Figures

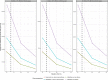
References
-
- Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, et al. . Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372:407–417. doi: 10.1056/NEJMoa1404595 - PubMed
-
- Magee LA, von Dadelszen P, Singer J, Lee T, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, et al. ; CHIPS Study Group*. The CHIPS Randomized Controlled Trial (Control of Hypertension in Pregnancy Study): is severe hypertension just an elevated blood pressure? Hypertension. 2016;68:1153–1159. doi: 10.1161/HYPERTENSIONAHA.116.07862 - PMC - PubMed
-
- National Institute for Health and Care Excellence (UK). Hypertension in pregnancy: diagnosis and management [Internet]. 2019. Available at: https://www.nice.org.uk/guidance/ng133 - PubMed
-
- WHO. WHO recommendations on drug treatment for non-severe hypertension in pregnancy. [Internet]. 2020. Geneva: Available at: https://www.who.int/publications/i/item/9789240008793 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

