Changes in supramolecular organization of cyanobacterial thylakoid membrane complexes in response to far-red light photoacclimation
- PMID: 35138895
- PMCID: PMC8827656
- DOI: 10.1126/sciadv.abj4437
Changes in supramolecular organization of cyanobacterial thylakoid membrane complexes in response to far-red light photoacclimation
Abstract
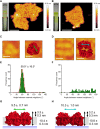
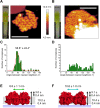
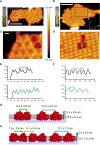
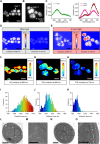
Cyanobacteria are ubiquitous in nature and have developed numerous strategies that allow them to live in a diverse range of environments. Certain cyanobacteria synthesize chlorophylls d and f to acclimate to niches enriched in far-red light (FRL) and incorporate paralogous photosynthetic proteins into their photosynthetic apparatus in a process called FRL-induced photoacclimation (FaRLiP). We characterized the macromolecular changes involved in FRL-driven photosynthesis and used atomic force microscopy to examine the supramolecular organization of photosystem I associated with FaRLiP in three cyanobacterial species. Mass spectrometry showed the changes in the proteome of Chroococcidiopsis thermalis PCC 7203 that accompany FaRLiP. Fluorescence lifetime imaging microscopy and electron microscopy reveal an altered cellular distribution of photosystem complexes and illustrate the cell-to-cell variability of the FaRLiP response.
Figures

Similar articles
-
Extensive remodeling of the photosynthetic apparatus alters energy transfer among photosynthetic complexes when cyanobacteria acclimate to far-red light.Biochim Biophys Acta Bioenerg. 2020 Apr 1;1861(4):148064. doi: 10.1016/j.bbabio.2019.148064. Epub 2019 Aug 14. Biochim Biophys Acta Bioenerg. 2020. PMID: 31421078
-
RfpA, RfpB, and RfpC are the Master Control Elements of Far-Red Light Photoacclimation (FaRLiP).Front Microbiol. 2015 Nov 25;6:1303. doi: 10.3389/fmicb.2015.01303. eCollection 2015. Front Microbiol. 2015. PMID: 26635768 Free PMC article.
-
Use of Quartz Sand Columns to Study Far-Red Light Photoacclimation (FaRLiP) in Cyanobacteria.Appl Environ Microbiol. 2022 Jul 12;88(13):e0056222. doi: 10.1128/aem.00562-22. Epub 2022 Jun 21. Appl Environ Microbiol. 2022. PMID: 35727027 Free PMC article.
-
Adaptive and acclimative responses of cyanobacteria to far-red light.Environ Microbiol. 2015 Oct;17(10):3450-65. doi: 10.1111/1462-2920.12992. Epub 2015 Aug 27. Environ Microbiol. 2015. PMID: 26234306 Review.
-
Light regulation of pigment and photosystem biosynthesis in cyanobacteria.Curr Opin Plant Biol. 2017 Jun;37:24-33. doi: 10.1016/j.pbi.2017.03.006. Epub 2017 Apr 6. Curr Opin Plant Biol. 2017. PMID: 28391049 Review.
Cited by
-
Lighting the way: Compelling open questions in photosynthesis research.Plant Cell. 2024 Oct 3;36(10):3914-3943. doi: 10.1093/plcell/koae203. Plant Cell. 2024. PMID: 39038210 Free PMC article.
-
Regulatory and retrograde signaling networks in the chlorophyll biosynthetic pathway.J Integr Plant Biol. 2025 Apr;67(4):887-911. doi: 10.1111/jipb.13837. Epub 2025 Jan 24. J Integr Plant Biol. 2025. PMID: 39853950 Free PMC article. Review.
-
A structure of the relict phycobilisome from a thylakoid-free cyanobacterium.Nat Commun. 2023 Dec 4;14(1):8009. doi: 10.1038/s41467-023-43646-9. Nat Commun. 2023. PMID: 38049400 Free PMC article.
-
Identification of far-red light acclimation in an endolithic Chroococcidiopsis strain and associated genomic features: Implications for oxygenic photosynthesis on exoplanets.Front Microbiol. 2022 Aug 4;13:933404. doi: 10.3389/fmicb.2022.933404. eCollection 2022. Front Microbiol. 2022. PMID: 35992689 Free PMC article.
-
Reflections on Cyanobacterial Chromatic Acclimation: Exploring the Molecular Bases of Organismal Acclimation and Motivation for Rethinking the Promotion of Equity in STEM.Microbiol Mol Biol Rev. 2022 Sep 21;86(3):e0010621. doi: 10.1128/mmbr.00106-21. Epub 2022 Jun 21. Microbiol Mol Biol Rev. 2022. PMID: 35727025 Free PMC article. Review.
References
-
- Flombaum P., Gallegos J. L., Gordillo R. A., Rincón J., Zabala L. L., Jiao N., Karl D. M., Li W. K., Lomas M. W., Veneziano D., Vera C. S., Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. U.S.A. 110, 9824–9829 (2013). - PMC - PubMed
-
- Garcia-Pichel F., Belnap J., Neuer S., Schanz F., Estimates of global cyanobacterial biomass and its distribution. Algol. Stud. 109, 213–227 (2003).
-
- D. A Bryant, The Molecular Biology of Cyanobacteria (Springer Science & Business Media, 1994), vol. 1.
-
- Kühl M., Chen M., Ralph P. J., Schreiber U., Larkum A. W., A niche for cyanobacteria containing chlorophyll d. Nature 433, 820 (2005). - PubMed
-
- Chen M., Schliep M., Willows R. D., Cai Z. L., Neilan B. A., Scheer H., A red-shifted chlorophyll. Science 329, 1318–1319 (2010). - PubMed

