Mutant glucocerebrosidase impairs α-synuclein degradation by blockade of chaperone-mediated autophagy
- PMID: 35138901
- PMCID: PMC11809618
- DOI: 10.1126/sciadv.abm6393
Mutant glucocerebrosidase impairs α-synuclein degradation by blockade of chaperone-mediated autophagy
Abstract
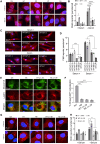
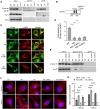
The most common genetic risk factors for Parkinson's disease (PD) are a set of heterozygous mutant (MT) alleles of the GBA1 gene that encodes β-glucocerebrosidase (GCase), an enzyme normally trafficked through the ER/Golgi apparatus to the lysosomal lumen. We found that half of the GCase in lysosomes from postmortem human GBA-PD brains was present on the lysosomal surface and that this mislocalization depends on a pentapeptide motif in GCase used to target cytosolic protein for degradation by chaperone-mediated autophagy (CMA). MT GCase at the lysosomal surface inhibits CMA, causing accumulation of CMA substrates including α-synuclein. Single-cell transcriptional analysis and proteomics of brains from GBA-PD patients confirmed reduced CMA activity and proteome changes comparable to those in CMA-deficient mouse brain. Loss of the MT GCase CMA motif rescued primary substantia nigra dopaminergic neurons from MT GCase-induced neuronal death. We conclude that MT GBA1 alleles block CMA function and produce α-synuclein accumulation.
Figures






References
-
- Cuervo A. M., Stefanis L., Fredenburg R., Lansbury P. T., Sulzer D., Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004). - PubMed
-
- Tang F. L., Erion J. R., Tian Y., Liu W., Yin D. M., Ye J., Tang B., Mei L., Xiong W. C., VPS35 in dopamine neurons is required for endosome-to-golgi retrieval of Lamp2a, a receptor of chaperone-mediated autophagy that is critical for α-synuclein degradation and prevention of pathogenesis of Parkinson’s disease. J. Neurosci. 35, 10613–10628 (2015). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

