Streptococcus pneumoniae colonization associates with impaired adaptive immune responses against SARS-CoV-2
- PMID: 35139037
- PMCID: PMC8970672
- DOI: 10.1172/JCI157124
Streptococcus pneumoniae colonization associates with impaired adaptive immune responses against SARS-CoV-2
Abstract
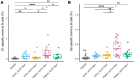
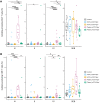
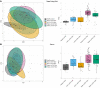
BackgroundAlthough recent epidemiological data suggest that pneumococci may contribute to the risk of SARS-CoV-2 disease, cases of coinfection with Streptococcus pneumoniae in patients with coronavirus disease 2019 (COVID-19) during hospitalization have been reported infrequently. This apparent contradiction may be explained by interactions of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and pneumococci in the upper airway, resulting in the escape of SARS-CoV-2 from protective host immune responses.MethodsHere, we investigated the relationship of these 2 respiratory pathogens in 2 distinct cohorts of health care workers with asymptomatic or mildly symptomatic SARS-CoV-2 infection identified by systematic screening and patients with moderate to severe disease who presented to the hospital. We assessed the effect of coinfection on host antibody, cellular, and inflammatory responses to the virus.ResultsIn both cohorts, pneumococcal colonization was associated with diminished antiviral immune responses, which primarily affected mucosal IgA levels among individuals with mild or asymptomatic infection and cellular memory responses in infected patients.ConclusionOur findings suggest that S. pneumoniae impair host immunity to SARS-CoV-2 and raise the question of whether pneumococcal carriage also enables immune escape of other respiratory viruses and facilitates reinfection.Trial registrationISRCTN89159899 (FASTER study) and ClinicalTrials.gov NCT03502291 (LAIV study).
Keywords: Adaptive immunity; Bacterial infections; Immunology; T cells; Virology.
Conflict of interest statement
Figures



References
-
- Gessner BD, et al. A post-hoc analysis of 13-valent pneumococcal conjugate vaccine efficacy against endemic human coronavirus-associated pneumonia. Paper presented at: The ESCMID Conference on Coronavirus Disease (ECCVID); September 23–25, 2020; Virtual. Accessed March 18, 2022.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

