Assessing Gq-GPCR-induced human astrocyte reactivity using bioengineered neural organoids
- PMID: 35139144
- PMCID: PMC8842185
- DOI: 10.1083/jcb.202107135
Assessing Gq-GPCR-induced human astrocyte reactivity using bioengineered neural organoids
Abstract
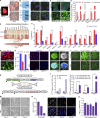
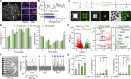
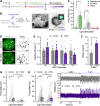
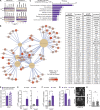
Astrocyte reactivity can directly modulate nervous system function and immune responses during disease and injury. However, the consequence of human astrocyte reactivity in response to specific contexts and within neural networks is obscure. Here, we devised a straightforward bioengineered neural organoid culture approach entailing transcription factor-driven direct differentiation of neurons and astrocytes from human pluripotent stem cells combined with genetically encoded tools for dual cell-selective activation. This strategy revealed that Gq-GPCR activation via chemogenetics in astrocytes promotes a rise in intracellular calcium followed by induction of immediate early genes and thrombospondin 1. However, astrocytes also undergo NF-κB nuclear translocation and secretion of inflammatory proteins, correlating with a decreased evoked firing rate of cocultured optogenetic neurons in suboptimal conditions, without overt neurotoxicity. Altogether, this study clarifies the intrinsic reactivity of human astrocytes in response to targeting GPCRs and delivers a bioengineered approach for organoid-based disease modeling and preclinical drug testing.
© 2022 Cvetkovic et al.
Figures






Comment in
-
Tweaking neural organoids to model human reactive astrocytes.J Cell Biol. 2022 Apr 4;221(4):e202202026. doi: 10.1083/jcb.202202026. Epub 2022 Mar 23. J Cell Biol. 2022. PMID: 35319768 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

