Withdrawal of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy
- PMID: 35139161
- PMCID: PMC9166547
- DOI: 10.1093/brain/awac054
Withdrawal of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy
Abstract
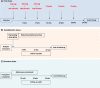
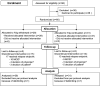
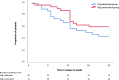
Intravenous immunoglobulins are an efficacious treatment for chronic inflammatory demyelinating polyradiculoneuropathy. Biomarkers for disease activity are lacking, making the need for ongoing treatment difficult to assess, leading to potential overtreatment and high health-care costs. Our objective was to determine whether intravenous immunoglobulin withdrawal is non-inferior to continuing intravenous immunoglobulin treatment and to determine how often patients are overtreated. We performed a randomized, double-blind, intravenous immunoglobulin-controlled non-inferiority trial in seven centres in the Netherlands (Trial registration: ISRCTN 13637698; www.isrctn.com/ISRCTN13637698). Adults with clinically stable chronic inflammatory demyelinating polyradiculoneuropathy using intravenous immunoglobulin maintenance treatment for at least 6 months were included. Patients received either intravenous immunoglobulin withdrawal (placebo) as investigational treatment or continuation of intravenous immunoglobulin treatment (control). The primary outcome was the mean change in logit scores from baseline to 24-week follow-up on the patient-reported Inflammatory Rasch-Overall Disability Scale. The non-inferiority margin was predefined as between-group difference in mean change scores of -0.65. Patients who deteriorated could reach a relapse end point according to predefined criteria. Patients with a relapse end point after intravenous immunoglobulin withdrawal entered a restabilization phase. All patients from the withdrawal group who remained stable were included in an open-label extension phase of 52 weeks. We included 60 patients, of whom 29 were randomized to intravenous immunoglobulin withdrawal and 31 to continuation of treatment. The mean age was 58 years (SD 14.7) and 67% was male. The between-group difference in mean change Inflammatory Rasch-Overall Disability Scale scores was -0.47 (95% CI -1.24 to 0.31), indicating that non-inferiority of intravenous immunoglobulin withdrawal could not be established. In the intravenous immunoglobulin withdrawal group, 41% remained stable for 24 weeks, compared to 58% in the intravenous immunoglobulin continuation group (-17%; 95% CI -39 to 8). Of the intravenous immunoglobulin withdrawal group, 28% remained stable at the end of the extension phase. Of the patients in the restabilization phase, 94% restabilized within 12 weeks. In conclusion, it remains inconclusive whether intravenous immunoglobulin withdrawal is non-inferior compared to continuing treatment, partly due to larger than expected confidence intervals leading to an underpowered study. Despite these limitations, a considerable proportion of patients could stop treatment and almost all patients who relapsed were restabilized quickly. Unexpectedly, a high proportion of intravenous immunoglobulin-treated patients experienced a relapse end point, emphasizing the need for more objective measures for disease activity in future trials, as the patient-reported outcome measures might not have been able to identify true relapses reliably. Overall, this study suggests that withdrawal attempts are safe and should be performed regularly in clinically stable patients.
Keywords: CIDP; IVIg; overtreatment; withdrawal.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Van den Bergh PY, Hadden RD, Bouche P, et al. . European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral nerve society. Eur J Neurol. 2010;17(3):356–363. - PubMed
-
- Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 2013;(12):CD001797. - PubMed
-
- van Schaik IN, Bril V, van Geloven N, et al. . Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35–46. - PubMed
-
- Hughes RAC, Donofrio P, Bril V, et al. . Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–144. - PubMed
-
- Nobile-Orazio E, Cocito D, Jann S, et al. . Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: A randomised controlled trial. Lancet Neurol. 2012;11(6):493–502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

