Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S
- PMID: 35139271
- PMCID: PMC8849184
- DOI: 10.1056/NEJMoa2117608
Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S
Abstract
Background: The Ad26.COV2.S vaccine was highly effective against severe-critical coronavirus disease 2019 (Covid-19), hospitalization, and death in the primary phase 3 efficacy analysis.
Methods: We conducted the final analysis in the double-blind phase of our multinational, randomized, placebo-controlled trial, in which adults were assigned in a 1:1 ratio to receive single-dose Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe-critical Covid-19 with onset at least 14 days after administration and at least 28 days after administration in the per-protocol population. Safety and key secondary and exploratory end points were also assessed.
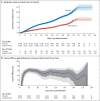
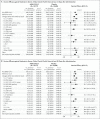
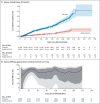
Results: Median follow-up in this analysis was 4 months; 8940 participants had at least 6 months of follow-up. In the per-protocol population (39,185 participants), vaccine efficacy against moderate to severe-critical Covid-19 at least 14 days after administration was 56.3% (95% confidence interval [CI], 51.3 to 60.8; 484 cases in the vaccine group vs. 1067 in the placebo group); at least 28 days after administration, vaccine efficacy was 52.9% (95% CI, 47.1 to 58.1; 433 cases in the vaccine group vs. 883 in the placebo group). Efficacy in the United States, primarily against the reference strain (B.1.D614G) and the B.1.1.7 (alpha) variant, was 69.7% (95% CI, 60.7 to 76.9); efficacy was reduced elsewhere against the P.1 (gamma), C.37 (lambda), and B.1.621 (mu) variants. Efficacy was 74.6% (95% CI, 64.7 to 82.1) against severe-critical Covid-19 (with only 4 severe-critical cases caused by the B.1.617.2 [delta] variant), 75.6% (95% CI, 54.3 to 88.0) against Covid-19 leading to medical intervention (including hospitalization), and 82.8% (95% CI, 40.5 to 96.8) against Covid-19-related death, with protection lasting 6 months or longer. Efficacy against any severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was 41.7% (95% CI, 36.3 to 46.7). Ad26.COV2.S was associated with mainly mild-to-moderate adverse events, and no new safety concerns were identified.
Conclusions: A single dose of Ad26.COV2.S provided 52.9% protection against moderate to severe-critical Covid-19. Protection varied according to variant; higher protection was observed against severe Covid-19, medical intervention, and death than against other end points and lasted for 6 months or longer. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722.).
Copyright © 2022 Massachusetts Medical Society.
Figures
References
-
- Nauta J. Statistics in clinical vaccine trials. New York: Springer-Verlag, 2011.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
- UM1 AI148684/NH/NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- U01 AI122285/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI68619/NH/NIH HHS/United States
- R01 AI161175/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI68635/NH/NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- S10 AI174104/AI/NIAID NIH HHS/United States
- UM1 AI148373/NH/NIH HHS/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- UM1 AI148685/NH/NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI68618/NH/NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI148452/NH/NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI68614/NH/NIH HHS/United States
- UM1 AI68636/NH/NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI148576/NH/NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
