Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation
- PMID: 35139352
- PMCID: PMC8852844
- DOI: 10.1016/j.immuni.2021.12.014
Interferon gamma constrains type 2 lymphocyte niche boundaries during mixed inflammation
Abstract
Allergic immunity is orchestrated by group 2 innate lymphoid cells (ILC2s) and type 2 helper T (Th2) cells prominently arrayed at epithelial- and microbial-rich barriers. However, ILC2s and Th2 cells are also present in fibroblast-rich niches within the adventitial layer of larger vessels and similar boundary structures in sterile deep tissues, and it remains unclear whether they undergo dynamic repositioning during immune perturbations. Here, we used thick-section quantitative imaging to show that allergic inflammation drives invasion of lung and liver non-adventitial parenchyma by ILC2s and Th2 cells. However, during concurrent type 1 and type 2 mixed inflammation, IFNγ from broadly distributed type 1 lymphocytes directly blocked both ILC2 parenchymal trafficking and subsequent cell survival. ILC2 and Th2 cell confinement to adventitia limited mortality by the type 1 pathogen Listeria monocytogenes. Our results suggest that the topography of tissue lymphocyte subsets is tightly regulated to promote appropriately timed and balanced immunity.
Keywords: 3D imaging; ILC2; Th2; allergic immunity; interferon gamma; lymphocyte niches; mixed inflammation; tissue immunology; type 2 immunity.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

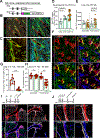
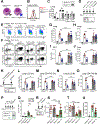




Comment in
-
IFN-γ(g)uarding the niche-Keeping ILC2 in check.Immunity. 2022 Feb 8;55(2):190-192. doi: 10.1016/j.immuni.2022.01.007. Immunity. 2022. PMID: 35139347
References
-
- Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, Fonseca R, Burbach BJ, Hickman HD, Vezys V, et al. (2018). Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory article. Nat. Immunol. 19, 173–182. - PMC - PubMed
-
- Bi J, Cui L, Yu G, Yang X, Chen Y, and Wan X (2017). NK Cells Alleviate Lung Inflammation by Negatively Regulating Group 2 Innate Lymphoid Cells. J. Immunol. 198, 3336–3344. - PubMed

