Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway
- PMID: 35139355
- PMCID: PMC8822620
- DOI: 10.1016/j.immuni.2022.01.003
Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway
Abstract
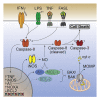
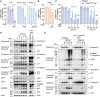
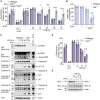
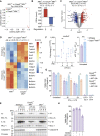
Cell death plays an important role during pathogen infections. Here, we report that interferon-γ (IFNγ) sensitizes macrophages to Toll-like receptor (TLR)-induced death that requires macrophage-intrinsic death ligands and caspase-8 enzymatic activity, which trigger the mitochondrial apoptotic effectors, BAX and BAK. The pro-apoptotic caspase-8 substrate BID was dispensable for BAX and BAK activation. Instead, caspase-8 reduced pro-survival BCL-2 transcription and increased inducible nitric oxide synthase (iNOS), thus facilitating BAX and BAK signaling. IFNγ-primed, TLR-induced macrophage killing required iNOS, which licensed apoptotic caspase-8 activity and reduced the BAX and BAK inhibitors, A1 and MCL-1. The deletion of iNOS or caspase-8 limited SARS-CoV-2-induced disease in mice, while caspase-8 caused lethality independent of iNOS in a model of hemophagocytic lymphohistiocytosis. These findings reveal that iNOS selectively licenses programmed cell death, which may explain how nitric oxide impacts disease severity in SARS-CoV-2 infection and other iNOS-associated inflammatory conditions.
Keywords: BAX and BAK; COVID-19; SARS-CoV-2; TNF; Toll-like receptor; apoptosis; caspase-8; hemophagocytic lymphohistiocytosis; iNOS; interferon.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that D.S.S., J.P., A.W., I.Y.K., M.R., J.P.C., K.C.D., S.H., H.A., M.D., Y.D., L.M., M.D., A.S.H., S.E.N., J.R.G., M.J.H., E.D.H., A.S., J.S., M.P., R.F., and J.E.V. are employees or former employees of the Walter and Eliza Hall Medical Institute, which receives milestone payments from Genentech and AbbVie for the development of ABT-199 for cancer therapy. J.E.V. sits on the advisory board of Avammune Therapeutics.
Figures

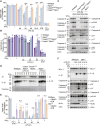
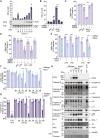

Comment in
-
The domiNO effect turns macrophage activation deadly.Immunity. 2022 Mar 8;55(3):382-384. doi: 10.1016/j.immuni.2022.02.010. Immunity. 2022. PMID: 35263563 Free PMC article.
References
-
- Allam R., Lawlor K.E., Yu E.C., Mildenhall A.L., Moujalled D.M., Lewis R.S., Ke F., Mason K.D., White M.J., Stacey K.J., et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–990. doi: 10.15252/embr.201438463. - DOI - PMC - PubMed
-
- Alvarez-Diaz S., Dillon C.P., Lalaoui N., Tanzer M.C., Rodriguez D.A., Lin A., Lebois M., Hakem R., Josefsson E.C., O’Reilly L.A., et al. The Pseudokinase MLKL and the Kinase RIPK3 Have Distinct Roles in Autoimmune Disease Caused by Loss of Death-Receptor-Induced Apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. - DOI - PMC - PubMed
-
- Bossaller L., Chiang P.I., Schmidt-Lauber C., Ganesan S., Kaiser W.J., Rathinam V.A., Mocarski E.S., Subramanian D., Green D.R., Silverman N., et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

