Dissecting Fc signatures of protection in neonates following maternal influenza vaccination in a placebo-controlled trial
- PMID: 35139373
- PMCID: PMC9026287
- DOI: 10.1016/j.celrep.2022.110337
Dissecting Fc signatures of protection in neonates following maternal influenza vaccination in a placebo-controlled trial
Abstract
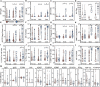
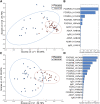
Influenza is an important cause of illness and morbidity for infants. Seasonal influenza vaccination during pregnancy aims to provide protection to mothers, but it can also provide immunity to infants. The precise influence of maternal vaccination on immunity in infants and how vaccine-elicited antibodies provide protection in some but not all infants is incompletely understood. We comprehensively profiled the transfer of functional antibodies and defined humoral factors contributing to immunity against influenza in a clinical trial of maternal influenza vaccination. Influenza-specific antibody subclass levels, Fc ɣ receptor (FCGR) binding levels, and antibody-dependent innate immune functions were all profiled in the mothers during pregnancy and at birth, as well as in cord blood. Vaccination increased influenza-specific antibody levels, antibody binding to FCGR, and specific antibody-dependent innate immune functions in both maternal and cord blood, with FCGR binding most enhanced via vaccination. Influenza-specific FCGR binding levels were lower in cord blood of infants who subsequently developed influenza infection. Collectively these data suggest that in addition to increased antibody amounts, the selective transfer of FCGR-binding antibodies contributes to the protective immune response in infants against influenza.
Keywords: Fc effector function; Fc receptor; adaptive immunity; antibody; influenza; innate immunity; maternal vaccination; placental transfer; vaccination.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.A. is a founder of SeromYx Systems, Inc.
Figures





References
-
- Akbulut H., Çelik A., Akbulut A., Ayar A. Placental transfer of total IgG and IgG subclasses in a Turkis population living in eastern Anatolia. Nobel Med. 2012;8:59–64.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

