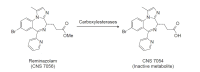Remimazolam: pharmacological characteristics and clinical applications in anesthesiology
- PMID: 35139608
- PMCID: PMC8841266
- DOI: 10.17085/apm.21115
Remimazolam: pharmacological characteristics and clinical applications in anesthesiology
Abstract
A novel ultra-short-acting benzodiazepine (BDZ), remimazolam (CNS 7056), has been designed by 'soft drug' development to achieve a better sedative profile than that of the current drugs. Notably, the esterase linkage in remimazolam permits rapid hydrolysis to inactivate metabolites by non-specific tissue esterase and induces a unique and favorable pharmacological profile, including rapid onset and offset of sedation and a predictable duration of action. Similar to other BDZs, its sedative effects can be reversed using flumazenil, a BDZ antagonist. The pharmacokinetics and pharmacodynamics of remimazolam are characterized by relatively high clearance, small steady-state volume of distribution, short elimination half-life, short context-sensitive half-life, and fast onset and recovery, indicating rapid elimination, minimal tissue accumulation, and good control. In addition, remimazolam possesses a superior safety profile, including low liability for cardiorespiratory depression and injection pain, making it a preferred hypnotic agent in various clinical settings. Early clinical investigations suggest that remimazolam is well tolerated and effective for procedural sedation and for induction and maintenance of general anesthesia. To date, however, the clinical use of remimazolam has been confined to a few volunteer studies and a limited number of clinical investigations. Therefore, further studies regarding its recovery issues or postoperative complications, characteristics of electroencephalogram changes, and cost-benefit analyses are required to facilitate its widespread use.
Keywords: Benzodiazepine; Hypnotic; Pharmacodynamics anesthesia; Pharmacokinetics; Remimazolam; Sedation.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci 1997; 18: 425-9. Erratum in: Trends Pharmacol Sci. 1998;19:256. - PubMed
-
- Egan TD. Is anesthesiology going soft?: trends in fragile pharmacology. Anesthesiology. 2009;111:229–30. - PubMed
-
- Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60–6. - PubMed
Publication types
LinkOut - more resources
Full Text Sources