Adenosine A1 receptor ligands bind to α-synuclein: implications for α-synuclein misfolding and α-synucleinopathy in Parkinson's disease
- PMID: 35139916
- PMCID: PMC8830172
- DOI: 10.1186/s40035-022-00284-3
Adenosine A1 receptor ligands bind to α-synuclein: implications for α-synuclein misfolding and α-synucleinopathy in Parkinson's disease
Abstract
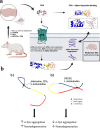
Background: Accumulating α-synuclein (α-syn) aggregates in neurons and glial cells are the staples of many synucleinopathy disorders, such as Parkinson's disease (PD). Since brain adenosine becomes greatly elevated in ageing brains and chronic adenosine A1 receptor (A1R) stimulation leads to neurodegeneration, we determined whether adenosine or A1R receptor ligands mimic the action of known compounds that promote α-syn aggregation (e.g., the amphetamine analogue 2-aminoindan) or inhibit α-syn aggregation (e.g., Rasagiline metabolite 1-aminoindan). In the present study, we determined whether adenosine, A1R receptor agonist N6-Cyclopentyladenosine (CPA) and antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) could directly interact with α-syn to modulate α-syn aggregation and neurodegeneration of dopaminergic neurons in the substantia nigra (SN).
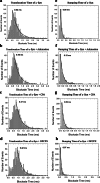
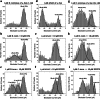
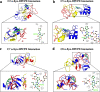
Methods: Nanopore analysis and molecular docking were used to test the binding properties of CPA and DPCPX with α-syn in vitro. Sprague-Dawley rats were administered with 7-day intraperitoneal injections of the A1R ligands and 1- and 2-aminoindan, and levels of α-syn aggregation and neurodegeneration were examined in the SN pars compacta and hippocampal regions using confocal imaging and Western blotting.
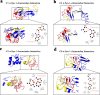
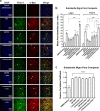
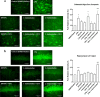
Results: Using nanopore analysis, we showed that the A1R agonists (CPA and adenosine) interacted with the N-terminus of α-syn, similar to 2-aminoindan, which is expected to promote a "knot" conformation and α-syn misfolding. In contrast, the A1R antagonist DPCPX interacted with the N- and C-termini of α-syn, similar to 1-aminoindan, which is expected to promote a "loop" conformation that prevents α-syn misfolding. Molecular docking studies revealed that adenosine, CPA and 2-aminoindan interacted with the hydrophobic core of α-syn N-terminus, whereas DPCPX and 1-aminoindan showed direct binding to the N- and C-terminal hydrophobic pockets. Confocal imaging and Western blot analyses revealed that chronic treatments with CPA alone or in combination with 2-aminoindan increased α-syn expression/aggregation and neurodegeneration in both SN pars compacta and hippocampus. In contrast, DPCPX and 1-aminoindan attenuated the CPA-induced α-syn expression/aggregation and neurodegeneration in SN and hippocampus.
Conclusions: The results indicate that A1R agonists and drugs promoting a "knot" conformation of α-syn can cause α-synucleinopathy and increase neuronal degeneration, whereas A1R antagonists and drugs promoting a "loop" conformation of α-syn can be harnessed for possible neuroprotective therapies to decrease α-synucleinopathy in PD.
Keywords: 1-aminoindan; 2-aminoindan; 8-cyclopentyl-1,3-dipropylxanthine; Adenosine A1 receptor; Alpha-synucleinopathy; N6-cyclopentyladenosine; Neurodegeneration; Neuroprotection; Protein misfolding.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
The Bifunctional Dimer Caffeine-Indan Attenuates α-Synuclein Misfolding, Neurodegeneration and Behavioral Deficits after Chronic Stimulation of Adenosine A1 Receptors.Int J Mol Sci. 2024 Aug 29;25(17):9386. doi: 10.3390/ijms25179386. Int J Mol Sci. 2024. PMID: 39273333 Free PMC article.
-
Monoamine Oxidase-B Inhibition Facilitates α-Synuclein Secretion In Vitro and Delays Its Aggregation in rAAV-Based Rat Models of Parkinson's Disease.J Neurosci. 2021 Sep 1;41(35):7479-7491. doi: 10.1523/JNEUROSCI.0476-21.2021. Epub 2021 Jul 21. J Neurosci. 2021. PMID: 34290084 Free PMC article.
-
Long-term adenosine A1 receptor activation-induced sortilin expression promotes α-synuclein upregulation in dopaminergic neurons.Neural Regen Res. 2020 Apr;15(4):712-723. doi: 10.4103/1673-5374.266916. Neural Regen Res. 2020. PMID: 31638096 Free PMC article.
-
The contribution of alpha synuclein to neuronal survival and function - Implications for Parkinson's disease.J Neurochem. 2016 May;137(3):331-59. doi: 10.1111/jnc.13570. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26852372 Free PMC article. Review.
-
Bent out of shape: α-Synuclein misfolding and the convergence of pathogenic pathways in Parkinson's disease.FEBS Lett. 2015 Dec 21;589(24 Pt A):3749-59. doi: 10.1016/j.febslet.2015.10.023. Epub 2015 Oct 23. FEBS Lett. 2015. PMID: 26505673 Free PMC article. Review.
Cited by
-
Activation of hepatic adenosine A1 receptor ameliorates MASH via inhibiting SREBPs maturation.Cell Rep Med. 2024 Mar 19;5(3):101477. doi: 10.1016/j.xcrm.2024.101477. Cell Rep Med. 2024. PMID: 38508143 Free PMC article.
-
The Bifunctional Dimer Caffeine-Indan Attenuates α-Synuclein Misfolding, Neurodegeneration and Behavioral Deficits after Chronic Stimulation of Adenosine A1 Receptors.Int J Mol Sci. 2024 Aug 29;25(17):9386. doi: 10.3390/ijms25179386. Int J Mol Sci. 2024. PMID: 39273333 Free PMC article.
-
The Anti-Parkinsonian A2A Receptor Antagonist Istradefylline (KW-6002) Attenuates Behavioral Abnormalities, Neuroinflammation, and Neurodegeneration in Cerebral Ischemia: An Adenosinergic Signaling Link Between Stroke and Parkinson's Disease.Int J Mol Sci. 2025 Jun 13;26(12):5680. doi: 10.3390/ijms26125680. Int J Mol Sci. 2025. PMID: 40565142 Free PMC article.
-
Tau Pathology Drives Disease-Associated Astrocyte Reactivity in Salt-Induced Neurodegeneration.Adv Sci (Weinh). 2025 Mar;12(11):e2410799. doi: 10.1002/advs.202410799. Epub 2025 Jan 24. Adv Sci (Weinh). 2025. PMID: 39853966 Free PMC article.
-
Adenosinergic Pathway in Parkinson's Disease: Recent Advances and Therapeutic Perspective.Mol Neurobiol. 2023 Jun;60(6):3054-3070. doi: 10.1007/s12035-023-03257-3. Epub 2023 Feb 14. Mol Neurobiol. 2023. PMID: 36786912 Review.
References
-
- Gillis P, Malter JS. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991;266(5):3172–3177. - PubMed
-
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. - PubMed
-
- Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34(1):5–14. - PubMed
-
- Cao X, Li LP, Qin XH, Li SJ, Zhang M, Wang Q, et al. Astrocytic Adenosine 5′-Triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells. 2013;31(8):1633–1643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

