A Neutralizing IL-11 Antibody Improves Renal Function and Increases Lifespan in a Mouse Model of Alport Syndrome
- PMID: 35140116
- PMCID: PMC8970448
- DOI: 10.1681/ASN.2021040577
A Neutralizing IL-11 Antibody Improves Renal Function and Increases Lifespan in a Mouse Model of Alport Syndrome
Abstract
Background: Alport syndrome is a genetic disorder characterized by a defective glomerular basement membrane, tubulointerstitial fibrosis, inflammation, and progressive renal failure. IL-11 was recently implicated in fibrotic kidney disease, but its role in Alport syndrome is unknown.
Methods: We determined IL-11 expression by molecular analyses and in an Alport syndrome mouse model. We assessed the effects of a neutralizing IL-11 antibody (×203) versus an IgG control in Col4a3-/- mice (lacking the gene encoding a type IV collagen component) on renal tubule damage, function, fibrosis, and inflammation. Effects of ×203, the IgG control, an angiotensin-converting enzyme (ACE) inhibitor (ramipril), or ramipril+X203 on lifespan were also studied.
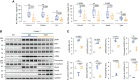
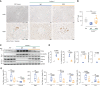
Results: In Col4a3-/- mice, as kidney failure advanced, renal IL-11 levels increased, and IL-11 expression localized to tubular epithelial cells. The IL-11 receptor (IL-11RA1) is expressed in tubular epithelial cells and podocytes and is upregulated in tubular epithelial cells of Col4a3-/- mice. Administration of ×203 reduced albuminuria, improved renal function, and preserved podocyte numbers and levels of key podocyte proteins that are reduced in Col4a3-/- mice; these effects were accompanied by reduced fibrosis and inflammation, attenuation of epithelial-to-mesenchymal transition, and increased expression of regenerative markers. X203 attenuated pathogenic ERK and STAT3 pathways, which were activated in Col4a3-/- mice. The median lifespan of Col4a3-/- mice was prolonged 22% by ramipril, 44% with ×203, and 99% with ramipril+X203.
Conclusions: In an Alport syndrome mouse model, renal IL-11 is upregulated, and neutralization of IL-11 reduces epithelial-to-mesenchymal transition, fibrosis, and inflammation while improving renal function. Anti-IL-11 combined with ACE inhibition synergistically extends lifespan. This suggests that a therapeutic approach targeting IL-11 holds promise for progressive kidney disease in Alport syndrome.
Keywords: Alport syndrome; chronic kidney disease; fibrosis; glomerular disease; glomerulosclerosis; interleukin 11; podocyte; therapy.
Copyright © 2022 by the American Society of Nephrology.
Figures






References
-
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG: Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348: 2543–2556, 2003 - PubMed
-
- Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 268: 26033–26036, 1993 - PubMed
-
- Jais JP, Knebelmann B, Giatras I, Marchi M, Rizzoni G, Renieri A, et al. : X-linked Alport syndrome: Natural history in 195 families and genotype–phenotype correlations in males. J Am Soc Nephrol 11: 649–657, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

