Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil
- PMID: 35140406
- PMCID: PMC9018414
- DOI: 10.1038/s41591-022-01701-w
Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil
Abstract
There is considerable interest in the waning of effectiveness of coronavirus disease 2019 (COVID-19) vaccines and vaccine effectiveness (VE) of booster doses. Using linked national Brazilian databases, we undertook a test-negative design study involving almost 14 million people (~16 million tests) to estimate VE of CoronaVac over time and VE of BNT162b2 booster vaccination against RT-PCR-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe COVID-19 outcomes (hospitalization or death). Compared with unvaccinated individuals, CoronaVac VE at 14-30 d after the second dose was 55.0% (95% confidence interval (CI): 54.3-55.7) against confirmed infection and 82.1% (95% CI: 81.4-82.8) against severe outcomes. VE decreased to 34.7% (95% CI: 33.1-36.2) against infection and 72.5% (95% CI: 70.9-74.0) against severe outcomes over 180 d after the second dose. A BNT162b2 booster, 6 months after the second dose of CoronaVac, improved VE against infection to 92.7% (95% CI: 91.0-94.0) and VE against severe outcomes to 97.3% (95% CI: 96.1-98.1) 14-30 d after the booster. Compared with younger age groups, individuals 80 years of age or older had lower protection after the second dose but similar protection after the booster. Our findings support a BNT162b2 booster vaccine dose after two doses of CoronaVac, particularly for the elderly.
© 2022. The Author(s).
Conflict of interest statement
S.V.K. was a member of the UK Government’s Scientific Advisory Group on Emergencies subgroup on ethnicity and the Cabinet Office’s International Best Practice Advisory Group and was co-chair of the Scottish Government’s Expert Reference Group on Ethnicity and COVID-19. C.R. reports grants from the Medical Research Council and Public Health Scotland during the conduct of the study and is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group, the Scientific Pandemic Influenza Group on Modelling and the Medicines and Healthcare Products Regulatory Agency Vaccine Benefit and Risk Working Group. A.S. is a member of the Scottish Government Chief Medical Officer’s COVID-19 Advisory Group and its Standing Committee on Pandemics. A.S. is also a member of the UK Government’s New and Emerging Respiratory Virus Threats Risk Stratification Subgroup. V.d.A.O., V.S.B., M.L.B. and M.B.-N. are employees of Fiocruz, a federal public institution, which manufactures Vaxzevria in Brazil through a full technology transfer agreement with AstraZeneca. Fiocruz allocates all its manufactured products to the Ministry of Health for the public health service use. All other authors declare no competing interests.
Figures

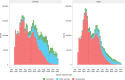

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

