Comparison of the Effectiveness and Tolerability of Nabiximols (THC:CBD) Oromucosal Spray versus Oral Dronabinol (THC) as Add-on Treatment for Severe Neuropathic Pain in Real-World Clinical Practice: Retrospective Analysis of the German Pain e-Registry
- PMID: 35140513
- PMCID: PMC8819705
- DOI: 10.2147/JPR.S340968
Comparison of the Effectiveness and Tolerability of Nabiximols (THC:CBD) Oromucosal Spray versus Oral Dronabinol (THC) as Add-on Treatment for Severe Neuropathic Pain in Real-World Clinical Practice: Retrospective Analysis of the German Pain e-Registry
Abstract
Purpose: To compare the effectiveness and tolerability of add-on treatment with nabiximols (NBX: delta-9-tetrahydrocannabinol: cannabidiol) oromucosal spray or oral dronabinol (DRO: synthetic tetrahydrocannabinol) in patients with severe neuropathic pain poorly responsive to established treatments.
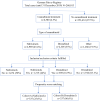
Methods: An analysis was conducted of anonymized, propensity score-matched real-world data from the German Pain e-Registry, using a sequential non-inferiority superiority approach, for adult outpatients with neuropathic pain who had initiated treatment with NBX or DRO between 10 March 2017 and 31 December 2019. The primary effectiveness variable was percent change from baseline in a 9-factor aggregated symptom relief (ASR-9) score, a composite index of nine distinct pain- and health-related parameters assessed using validated patient-reported instruments. Safety was assessed by the incidence of physician-confirmed treatment-related adverse events (TRAEs), and TRAEs leading to discontinuation.
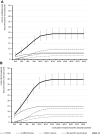
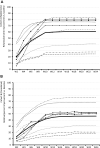
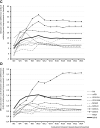
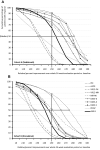
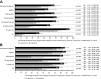
Results: Propensity score-matched data were analyzed for 337 patients treated with NBX and 337 patients treated with DRO. Mean (standard deviation) THC dose over the 24-week evaluation period was 16.6 (6.5) mg for NBX and 17.2 (7.6) mg for DRO (p<0.001). Median (standard error) improvement relative to baseline in the ASR-9 composite score was 55.4% (0.5) for NBX and 40.5% (0.5) for DRO (least squares mean difference, 14.0 (0.7), 95% confidence interval 12.6-15.4; p<0.001), and incidences of TRAEs (21.1 vs 35%) and TRAE-related discontinuations (5.9 vs 14.8%) were significantly lower with NBX than DRO (p<0.001 for both), collectively indicating pre-specified non-inferiority and superiority of NBX. More NBX- than DRO-treated patients discontinued non-cannabinoid background pain medications and rescue analgesics, especially opioid analgesics (p<0.001 for both).
Conclusion: Add-on treatment with cannabinoids is effective for treatment of severe neuropathic pain with inadequate response to established treatments. In daily practice, NBX had superior effectiveness and tolerability compared to DRO. The results emphasize the importance of combining CBD with THC in this patient population.
Keywords: German Pain e-Registry; dronabinol; nabiximols; neuropathic pain; oromucosal spray; real-world data.
© 2022 Ueberall et al.
Conflict of interest statement
MAU has received financial support and/or expenses in form of research funds, consultancy fees and/or renumerations for lecture activities from Allergan, Almirall, Amicus Therapeutics, Aristo Pharma, Bionorica, Glaxo Smith Kline, Grünenthal, Hapa Medical, Hexal, IMC, Kyowa-Kirin, Labatec, Mucos, Mundipharma, Nestle, Pfizer, Recordati, Servier, SGP-Pharma, Shionogi, Spectrum Therapeutics, Strathmann, Teva, and Tilray. He is also an honorary member of the management boards of the German Pain Association and the German Pain League, Medical Director of the private Institute of Neurological Sciences and CEO of O.Meany-MDPM GmbH, all of which are working on/with the German Pain e-Registry. UE has received honoraria for consultancy services from Almirall Hermal GmbH and Granzer Regulatory Consulting & Service. CVS is a full-time employee of Almirall S.A. GHHM-S has received financial support and/or expenses in the form of research funds, consultancy fees and/or remunerations for lecture activities from Allergan, Almirall, Grünenthal, Mundipharma, Pfizer, PharmAllergan, ProStrakan, and Teva. The authors report no other conflicts of interest in this work.
Figures


References
LinkOut - more resources
Full Text Sources

