Inhibition of renal fibrosis with a human CXCL9-derived glycosaminoglycan-binding peptide
- PMID: 35140938
- PMCID: PMC8810938
- DOI: 10.1002/cti2.1370
Inhibition of renal fibrosis with a human CXCL9-derived glycosaminoglycan-binding peptide
Abstract
Objectives: Renal fibrosis accompanies all chronic kidney disorders, ultimately leading to end-stage kidney disease and the need for dialysis or even renal replacement. As such, renal fibrosis poses a major threat to global health and the search for effective therapeutic strategies to prevent or treat fibrosis is highly needed. We evaluated the applicability of a highly positively charged human peptide derived from the COOH-terminal domain of the chemokine CXCL9, namely CXCL9(74-103), for therapeutic intervention. Because of its high density of net positive charges at physiological pH, CXCL9(74-103) competes with full-length chemokines for glycosaminoglycan (GAG) binding. Consequently, CXCL9(74-103) prevents recruitment of inflammatory leucocytes to sites of inflammation.
Methods: CXCL9(74-103) was chemically synthesised and tested in vitro for anti-fibrotic properties on human fibroblasts and in vivo in the unilateral ureteral obstruction (UUO) mouse model.
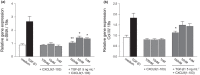
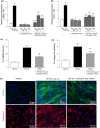
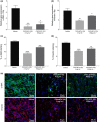
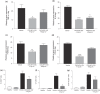
Results: CXCL9(74-103) significantly reduced the mRNA and/or protein expression of connective tissue growth factor (CTGF), alpha-smooth muscle actin (α-SMA) and collagen III by transforming growth factor (TGF)-β1-stimulated human fibroblasts. In addition, administration of CXCL9(74-103) inhibited fibroblast migration towards platelet-derived growth factor (PDGF), without affecting cell viability. In the UUO model, CXCL9(74-103) treatment significantly decreased renal α-SMA, vimentin, and fibronectin mRNA and protein expression. Compared with vehicle, CXCL9(74-103) attenuated mRNA expression of TGF-β1 and the inflammatory markers/mediators MMP-9, F4/80, CCL2, IL-6 and TNF-α. Finally, CXCL9(74-103) treatment resulted in reduced influx of leucocytes in the UUO model and preserved tubular morphology. The anti-fibrotic and anti-inflammatory effects of CXCL9(74-103) were mediated by competition with chemokines and growth factors for GAG binding.
Conclusions: Our findings provide a scientific rationale for targeting GAG-protein interactions in renal fibrotic disease.
Keywords: CXCL9; chemokine‐derived peptides; glycosaminoglycans; inflammation; renal fibrosis.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 2006; 69: 213–217. - PubMed
-
- Mack M. Inflammation and fibrosis. Matrix Biol 2018; 68–69: 106–121. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000; 12: 121–127. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
