Cancer immunomodulation using bispecific aptamers
- PMID: 35141049
- PMCID: PMC8803965
- DOI: 10.1016/j.omtn.2022.01.008
Cancer immunomodulation using bispecific aptamers
Abstract
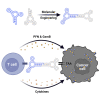
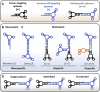
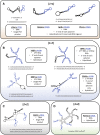
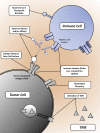
Evasion of immune destruction is a major hallmark of cancer. Recent US Food and Drug Administration (FDA) approvals of various immunomodulating therapies underline the important role that reprogramming the immune system can play in combating this disease. However, a wide range of side effects still limit the therapeutic potential of immunomodulators, suggesting a need for more precise reagents with negligible off-target and on-target/off-tumor effects. Aptamers are single-chained oligonucleotides that bind their targets with high specificity and affinity owing to their three-dimensional (3D) structures, and they are one potential way to address this need. In particular, bispecific aptamers (bsApts) have been shown to induce artificial immune synapses that promote T cell activation and subsequent tumor cell lysis in various in vitro and in vivo pre-clinical models. We discuss these advances here, along with gaps in bsApt biology at both the cellular and resident tissue levels that should be addressed to accelerate their translation into the clinic. The broad application, minimal production cost, and relative lack of immunogenicity of bsApts give them some ideal qualities for manipulating the immune system. Building upon lessons from other novel therapies, bsApts could soon provide clinicians with an immunomodulating toolbox that is not only potent and efficacious but exercises a wide therapeutic index.
Keywords: antibody; bispecific; clinical translation; immunomodulation; molecular design; oligonucleotide therapy; oncolytic.
© 2022 The Authors.
Figures

References
-
- Hulvat M.C. Cancer incidence and trends. Surg. Clin. North Am. 2020;100:469–481. - PubMed
-
- Torre L.A., Siegel R.L., Ward E.M., Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol. Biomark. Prev. 2016;25:16–27. - PubMed
-
- WHO | projections of mortality and causes of death, 2016 to 2060 WHO. http://www.who.int/healthinfo/global_burden_disease/projections/en/
-
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

