Development of Neuronal Guidance Fibers for Stimulating Electrodes: Basic Construction and Delivery of a Growth Factor
- PMID: 35141211
- PMCID: PMC8819688
- DOI: 10.3389/fbioe.2022.776890
Development of Neuronal Guidance Fibers for Stimulating Electrodes: Basic Construction and Delivery of a Growth Factor
Abstract
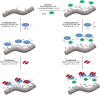
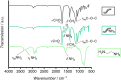
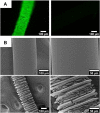
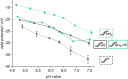
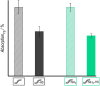
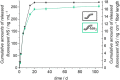
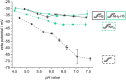
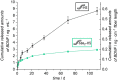
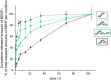
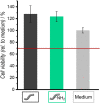
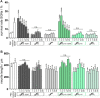
State-of-the-art treatment for sensorineural hearing loss is based on electrical stimulation of residual spiral ganglion neurons (SGNs) with cochlear implants (CIs). Due to the anatomical gap between the electrode contacts of the CI and the residual afferent fibers of the SGNs, spatial spreading of the stimulation signal hampers focused neuronal stimulation. Also, the efficiency of a CI is limited because SGNs degenerate over time due to loss of trophic support. A promising option to close the anatomical gap is to install fibers as artificial nerve guidance structures on the surface of the implant and install on these fibers drug delivery systems releasing neuroprotective agents. Here, we describe the first steps in this direction. In the present study, suture yarns made of biodegradable polymers (polyglycolide/poly-ε-caprolactone) serve as the basic fiber material. In addition to the unmodified fiber, also fibers modified with amine groups were employed. Cell culture investigations with NIH 3T3 fibroblasts attested good cytocompatibility to both types of fibers. The fibers were then coated with the extracellular matrix component heparan sulfate (HS) as a biomimetic of the extracellular matrix. HS is known to bind, stabilize, modulate, and sustainably release growth factors. Here, we loaded the HS-carrying fibers with the brain-derived neurotrophic factor (BDNF) which is known to act neuroprotectively. Release of this neurotrophic factor from the fibers was followed over a period of 110 days. Cell culture investigations with spiral ganglion cells, using the supernatants from the release studies, showed that the BDNF delivered from the fibers drastically increased the survival rate of SGNs in vitro. Thus, biodegradable polymer fibers with attached HS and loaded with BDNF are suitable for the protection and support of SGNs. Moreover, they present a promising base material for the further development towards a future neuronal guiding scaffold.
Keywords: BDNF; cochlea implant; heparan sulfate; implant-associated drug delivery; polycaprolactone; polyglycolide.
Copyright © 2022 Wille, Harre, Oehmichen, Lindemann, Menzel, Ehlert, Lenarz, Warnecke and Behrens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro.PLoS One. 2018 Mar 27;13(3):e0194778. doi: 10.1371/journal.pone.0194778. eCollection 2018. PLoS One. 2018. PMID: 29584754 Free PMC article.
-
Promoting neurite outgrowth from spiral ganglion neuron explants using polypyrrole/BDNF-coated electrodes.J Biomed Mater Res A. 2009 Oct;91(1):241-50. doi: 10.1002/jbm.a.32228. J Biomed Mater Res A. 2009. PMID: 18814235
-
Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss.J Comp Neurol. 2005 May 30;486(2):145-58. doi: 10.1002/cne.20564. J Comp Neurol. 2005. PMID: 15844207 Free PMC article.
-
Neurotrophins and cochlear implants: a solution to sensorineural deafness?Cochlear Implants Int. 2013 Jun;14(3):158-64. doi: 10.1179/1754762812Y.0000000013. Cochlear Implants Int. 2013. PMID: 22889496 Review.
-
Regulation of Spiral Ganglion Neuron Regeneration as a Therapeutic Strategy in Sensorineural Hearing Loss.Front Mol Neurosci. 2022 Jan 20;14:829564. doi: 10.3389/fnmol.2021.829564. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35126054 Free PMC article. Review.
Cited by
-
Recent progresses in neural tissue engineering using topographic scaffolds.Am J Stem Cells. 2024 Feb 25;13(1):1-26. doi: 10.62347/WMDZ8890. eCollection 2024. Am J Stem Cells. 2024. PMID: 38505822 Free PMC article. Review.
-
Tissue engineering strategies for spiral ganglion neuron protection and regeneration.J Nanobiotechnology. 2024 Jul 31;22(1):458. doi: 10.1186/s12951-024-02742-8. J Nanobiotechnology. 2024. PMID: 39085923 Free PMC article. Review.
-
Inositol trisphosphate and ryanodine receptor signaling distinctly regulate neurite pathfinding in response to engineered micropatterned surfaces.PLoS One. 2024 Sep 5;19(9):e0308389. doi: 10.1371/journal.pone.0308389. eCollection 2024. PLoS One. 2024. PMID: 39236043 Free PMC article.
-
Decreasing the physical gap in the neural-electrode interface and related concepts to improve cochlear implant performance.Front Neurosci. 2024 Jul 24;18:1425226. doi: 10.3389/fnins.2024.1425226. eCollection 2024. Front Neurosci. 2024. PMID: 39114486 Free PMC article. Review.
References
-
- Bajgai M. P., Kim K.-W., Chandra Parajuli D., Yoo Y. C., Kim W. D., Khil M.-S., et al. (2008). In Vitro hydrolytic Degradation of Poly(ɛ-Caprolactone) Grafted Dextran Fibers and Films. Polym. Degrad. Stab. 93, 2172–2179. 10.1016/j.polymdegradstab.2008.08.002 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

