Comparison of Tendon Development Versus Tendon Healing and Regeneration
- PMID: 35141224
- PMCID: PMC8819183
- DOI: 10.3389/fcell.2022.821667
Comparison of Tendon Development Versus Tendon Healing and Regeneration
Abstract
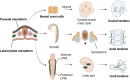
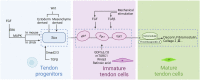
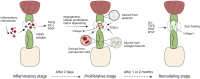
Tendon is a vital connective tissue in human skeletal muscle system, and tendon injury is very common and intractable in clinic. Tendon development and repair are two closely related but still not fully understood processes. Tendon development involves multiple germ layer, as well as the regulation of diversity transcription factors (Scx et al.), proteins (Tnmd et al.) and signaling pathways (TGFβ et al.). The nature process of tendon repair is roughly divided in three stages, which are dominated by various cells and cell factors. This review will describe the whole process of tendon development and compare it with the process of tendon repair, focusing on the understanding and recent advances in the regulation of tendon development and repair. The study and comparison of tendon development and repair process can thus provide references and guidelines for treatment of tendon injuries.
Keywords: comparison; development; healing; regeneration; regulation; tendon.
Copyright © 2022 He, Ruan, Huang, Wang, Xu, Cai, Liu, Fei, Heng, Chen and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Akiyama H., Chaboissier M.-C., Martin J. F., Schedl A., de Crombrugghe B. (2002). The Transcription Factor Sox9 Has Essential Roles in Successive Steps of the Chondrocyte Differentiation Pathway and Is Required for Expression of Sox5 and Sox6. Genes Dev. 16 (21), 2813–2828. 10.1101/gad.1017802 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

