Anatomical Site, Viral Ribonucleic Acid Abundance, and Time of Sampling Correlate With Molecular Detection of Severe Acute Respiratory Syndrome Coronavirus 2 During Infection
- PMID: 35141345
- PMCID: PMC8689744
- DOI: 10.1093/ofid/ofab623
Anatomical Site, Viral Ribonucleic Acid Abundance, and Time of Sampling Correlate With Molecular Detection of Severe Acute Respiratory Syndrome Coronavirus 2 During Infection
Abstract
Background: Nasopharyngeal (NP) swabs are the standard for SARS-CoV-2 diagnosis. If less invasive alternatives to NP swabs (eg, oropharyngeal [OP] or nasal swabs [NS]) are comparably sensitive, the use of these techniques may be preferable in terms of comfort, convenience, and safety.
Methods: This study compared the detection of SARS-CoV-2 in swab samples collected on the same day among participants with at least one positive PCR test.
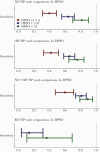
Results: Overall, 755 participants had at least one set of paired swabs. Concordance between NP and other swab types was 75% (NS), 72% (OP), 54% (rectal swabs [RS]), and 78% (NS/OP combined). Kappa values were moderate for the NS, OP, and NS/OP comparisons (0.50, 0.45, and 0.54, respectively). Highest sensitivity relative to NP (0.87) was observed with a combination of NS/OP tests (positive if either NS or OP was positive). Sensitivity of the non-NP swab types was highest in the first week postsymptom onset and decreased thereafter. Similarly, virus RNA quantity was highest in the NP swabs as compared with NS, OP, and RS within two weeks postsymptom onset. OP and NS performance decreased as virus RNA quantity decreased. No differences were noted between NS specimens collected at home or in clinic.
Conclusions: NP swabs detected more SARS-CoV-2 cases than non-NP swabs, and the sensitivity of the non-NP swabs decreased with time postsymptom onset. While other swabs may be simpler to collect, NP swabs present the best chance of detecting SARS-CoV-2 RNA, which is essential for clinical care as well as genomic surveillance.
Keywords: COVID-19; SARS-CoV-2; nasal swabs; nasopharyngeal; oropharyngeal.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2021.
Figures

References
-
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting and Handling of Clinical Specimens for Covid-19 Testing. Updated 25 October 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed 22 December 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

