Trans-seeding of Alzheimer-related tau protein by a yeast prion
- PMID: 35142027
- PMCID: PMC10078693
- DOI: 10.1002/alz.12581
Trans-seeding of Alzheimer-related tau protein by a yeast prion
Abstract
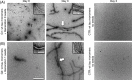
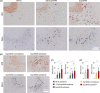
Abnormal tau protein aggregates constitute a hallmark of Alzheimer's disease. The mechanisms underlying the initiation of tau aggregation in sporadic neurodegeneration remain unclear. Here we investigate whether a non-human prion can seed tau aggregation. Due to their structural similarity with tau aggregates, we chose Sup35NM yeast prion domain fibrils for explorative tau seedings. Upon in vitro incubation with tau monomers, Sup35NM fibrils promoted the formation of morphologically distinct tau fibril strains. In vivo, intrahippocampal inoculation of Sup35NM fibrils accentuated tau pathology in P301S tau transgenic mice. Thus, our results provide first in vivo evidence for heterotypic cross-species seeding of a neurodegenerative human prion-like protein by a yeast prion. This opens up the conceptual perspective that non-mammalian prions present in the human microbiome could be involved in the initiation of protein misfolding in neurodegenerative disorders, a mechanism for which we propose the term "trans-seeding."
Keywords: Alzheimer's disease; Sup35; neurodegeneration; prion; seeding; tau; trans-seeding; yeast.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Goedert M . Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Abeta, tau, and alpha‐synuclein. Science. 2015;349:1255555. - PubMed
-
- Alzheimer's disease facts and figures. Alzheimer's Dementn. 2016;12:459‐509. - PubMed
-
- Arriagada PV, Growdon JH, Hedley‐Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631‐639. - PubMed
-
- Goedert M, Eisenberg DS, Crowther RA. Propagation of tau aggregates and neurodegeneration. Annu Rev Neurosci. 2017;40:189‐210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

