Application of dose-addition analyses to characterize the abuse-related effects of drug mixtures
- PMID: 35142382
- PMCID: PMC9327442
- DOI: 10.1002/jeab.741
Application of dose-addition analyses to characterize the abuse-related effects of drug mixtures
Abstract
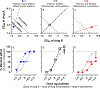
Polysubstance use makes up a majority of drug use, yet relatively few studies investigate the abuse-related effects of drug mixtures. Dose-addition analyses provide a rigorous and quantitative method to determine the nature of the interaction (i.e., supraadditive, additive, or subadditive) between two or more drugs. As briefly reviewed here, studies in rhesus monkeys have applied dose-addition analyses to group level data to characterize the nature of the interaction between the reinforcing effects of stimulants and opioids (e.g., mixtures of cocaine + heroin). Building upon these foundational studies, more recent work has applied dose-addition analyses to better understand the nature of the interaction between caffeine and illicit stimulants such as MDPV and methamphetamine in rats. In addition to utilizing a variety of operant procedures, including drug discrimination, drug self-administration, and drug-primed reinstatement, these studies have incorporated potency and effectiveness ratios as a method for both statistical analysis and visualization of departures from additivity at both the group and individual subject level. As such, dose-addition analyses represent a powerful and underutilized approach to quantify the nature of drug-drug interactions that can be applied to a variety of abuse-related endpoints in order to better understand the behavioral pharmacology of polysubstance use.
Keywords: dose-addition analyses; drug mixtures; self-administration.
© 2022 Society for the Experimental Analysis of Behavior.
Figures


References
-
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, & Taffe MA (2013). The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology, 71, 130–140. 10.1016/j.neuropharm.2013.04.003 - DOI - PMC - PubMed
-
- Arango-Meriño L, Quevedo-Castro C, Mancera-Barros J, Sarmiento-Gutiérrez ÁE, Arana VA, & Granados-Reyes J (2021). Cutting agents in cocaine: A temporal study of the period 2015–2017 in the Northern Region of Colombia. Forensic Science International, 327, 110911. 10.1016/J.FORSCIINT.2021.110911 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

