Remodeling Tumor-Associated Neutrophils to Enhance Dendritic Cell-Based HCC Neoantigen Nano-Vaccine Efficiency
- PMID: 35142445
- PMCID: PMC9009112
- DOI: 10.1002/advs.202105631
Remodeling Tumor-Associated Neutrophils to Enhance Dendritic Cell-Based HCC Neoantigen Nano-Vaccine Efficiency
Abstract
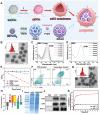
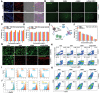
Hepatocellular carcinoma (HCC) commonly emerges in an immunologically "cold" state, thereafter protects it away from cytolytic attack by tumor-infiltrating lymphocytes, resulting in poor response to immunotherapy. Herein, an acidic/photo-sensitive dendritic cell (DCs)-based neoantigen nano-vaccine has been explored to convert tumor immune "cold" state into "hot", and remodel tumor-associated neutrophils to potentiate anticancer immune response for enhancing immunotherapy efficiency. The nano-vaccine is constructed by SiPCCl2 -hybridized mesoporous silica with coordination of Fe(III)-captopril, and coating with exfoliated membrane of matured DCs by H22-specific neoantigen stimulation. The nano-vaccines actively target H22 tumors and induce immunological cell death to boost tumor-associated antigen release by the generation of excess 1 O2 through photodynamic therapy, which act as in situ tumor vaccination to strengthen antitumor T-cell response against primary H22 tumor growth. Interestingly, the nano-vaccines are also home to lymph nodes to directly induce the activation and proliferation of neoantigen-specific T cells to suppress the primary/distal tumor growth. Moreover, the acidic-triggered captopril release in tumor microenvironment can polarize the protumoral N2 phenotype neutrophils to antitumor N1 phenotype for improving the immune effects to achieve complete tumor regression (83%) in H22-bearing mice and prolong the survival time. This work provides an alternative approach for developing novel HCC immunotherapy strategies.
Keywords: dendritic cell-based vaccine; immunotherapy; neoantigen; neutrophils; photodynamic therapy.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Caveolin-mediated cytosolic delivery of spike nanoparticle enhances antitumor immunity of neoantigen vaccine for hepatocellular carcinoma.Theranostics. 2023 Jul 16;13(12):4166-4181. doi: 10.7150/thno.85843. eCollection 2023. Theranostics. 2023. PMID: 37554274 Free PMC article.
-
Enhancement of antitumor vaccine in ablated hepatocellular carcinoma by high-intensity focused ultrasound.World J Gastroenterol. 2010 Jul 28;16(28):3584-91. doi: 10.3748/wjg.v16.i28.3584. World J Gastroenterol. 2010. PMID: 20653069 Free PMC article.
-
Immunotherapeutic strategies for treatment of hepatocellular carcinoma with antigen-loaded dendritic cells: in vivo study.Clin Exp Med. 2018 Nov;18(4):535-546. doi: 10.1007/s10238-018-0521-6. Epub 2018 Jul 30. Clin Exp Med. 2018. PMID: 30062618
-
Dendritic cell as therapeutic vaccines against tumors and its role in therapy for hepatocellular carcinoma.Cell Mol Immunol. 2006 Jun;3(3):197-203. Cell Mol Immunol. 2006. PMID: 16893500 Review.
-
Dendritic Cell and T Cell Crosstalk in Liver Fibrogenesis and Hepatocarcinogenesis: Implications for Prevention and Therapy of Liver Cancer.Int J Mol Sci. 2020 Oct 6;21(19):7378. doi: 10.3390/ijms21197378. Int J Mol Sci. 2020. PMID: 33036244 Free PMC article. Review.
Cited by
-
Mn(iii)-mediated carbon-centered radicals generate an enhanced immunotherapeutic effect.Chem Sci. 2023 Dec 6;15(2):765-777. doi: 10.1039/d3sc03635a. eCollection 2024 Jan 3. Chem Sci. 2023. PMID: 38179519 Free PMC article.
-
Research progress of nanovaccine in anti-tumor immunotherapy.Front Oncol. 2023 Aug 24;13:1211262. doi: 10.3389/fonc.2023.1211262. eCollection 2023. Front Oncol. 2023. PMID: 37692854 Free PMC article. Review.
-
The evolution and heterogeneity of neutrophils in cancers: origins, subsets, functions, orchestrations and clinical applications.Mol Cancer. 2023 Sep 7;22(1):148. doi: 10.1186/s12943-023-01843-6. Mol Cancer. 2023. PMID: 37679744 Free PMC article. Review.
-
Trends in research related to vaccine and cancer prevention from 1992 to 2022: A 30-years bibliometric analysis.Hum Vaccin Immunother. 2023 Dec 31;19(1):2207441. doi: 10.1080/21645515.2023.2207441. Epub 2023 May 9. Hum Vaccin Immunother. 2023. PMID: 37158187 Free PMC article.
-
Cancer stem cell-immune cell crosstalk in the tumor microenvironment for liver cancer progression.Front Med. 2024 Jun;18(3):430-445. doi: 10.1007/s11684-023-1049-z. Epub 2024 Apr 11. Front Med. 2024. PMID: 38600350 Review.
References
-
- Llovet J. M., Kelley R. K., Villanueva A., Singal A. G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman‐Rossi J., Finn R. S., Nat. Rre. Dis. Primers 2021, 7, 6. - PubMed
-
- Forner A., Gilabert M., Bruix J., Raoul J. L., Nat. Rev. Clin. Oncol. 2014, 11, 525. - PubMed
-
- Vogel A., Cervantes A., Chau I., Daniele B., Llovet J. M., Meyer T., Nault J. C., Neumann U., Ricke J., Sangro B., Schirmacher P., Verslype C., Zech C. J., Arnold D., Martinelli E., Ann. Oncol. 2019, 30, 871. - PubMed
-
- Llovet J. M., Montal R., Sia D., Finn R. S., Nat Rev Clin Oncol. 2018, 15, 599. - PubMed
-
- Llovet J. M., De Baere T., Kulik L., Haber P. K., Greten T. F., Meyer T., Lencioni R., Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 62175031/National Natural Science Foundation of China
- 61875141/National Natural Science Foundation of China
- 2020J011154/Natural Science Foundation of Fujian Province of China
- 2021-S-wp1/Scientific Foundation of Fuzhou Municipal Health commission
- 2021GGB020/Young and Middle-aged Talent Training Project of Fujian Provincial Health Commission
LinkOut - more resources
Full Text Sources
Medical
