Human embryoid bodies as a novel system for genomic studies of functionally diverse cell types
- PMID: 35142607
- PMCID: PMC8830892
- DOI: 10.7554/eLife.71361
Human embryoid bodies as a novel system for genomic studies of functionally diverse cell types
Abstract
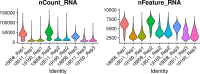
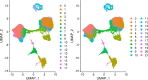
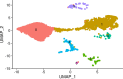
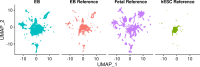
Practically all studies of gene expression in humans to date have been performed in a relatively small number of adult tissues. Gene regulation is highly dynamic and context-dependent. In order to better understand the connection between gene regulation and complex phenotypes, including disease, we need to be able to study gene expression in more cell types, tissues, and states that are relevant to human phenotypes. In particular, we need to characterize gene expression in early development cell types, as mutations that affect developmental processes may be of particular relevance to complex traits. To address this challenge, we propose to use embryoid bodies (EBs), which are organoids that contain a multitude of cell types in dynamic states. EBs provide a system in which one can study dynamic regulatory processes at an unprecedentedly high resolution. To explore the utility of EBs, we systematically explored cellular and gene expression heterogeneity in EBs from multiple individuals. We characterized the various cell types that arise from EBs, the extent to which they recapitulate gene expression in vivo, and the relative contribution of technical and biological factors to variability in gene expression, cell composition, and differentiation efficiency. Our results highlight the utility of EBs as a new model system for mapping dynamic inter-individual regulatory differences in a large variety of cell types.
Keywords: embryoid bodies; genetics; genomics; human; iPSC; scRNA-seq; single cell.
Plain language summary
One major goal of human genetics is to understand how changes in the way genes are regulated affect human traits, including disease susceptibility. To date, most studies of gene regulation have been performed in adult tissues, such as liver or kidney tissue, that were collected at a single time point. Yet, gene regulation is highly dynamic and context-dependent, meaning that it is important to gather data from a greater variety of cell types at different stages of their development. Additionally, observing which genes switch on and off in response to external treatments can shed light on how genetic variation can drive errors in gene regulation and cause diseases. Stem cells can produce more cells like themselves or differentiate – acquire the characteristics – of many cell types. These cells have been used in the laboratory to research gene regulation. Unfortunately, these studies often fail to capture the complex spatial and temporal dynamics of stem cell differentiation; in particular, these studies are unable to observe gene regulation in the transient cell types that appear early in embryonic development. To overcome these limitations, scientists developed systems such as embryoid bodies: three-dimensional aggregates of stem cells that, when grown under certain conditions, spontaneously develop into a variety of cell types. Rhodes, Barr et al. wanted to assess the utility of embryoid bodies as a model to study how genes are dynamically regulated in different cell types, by different individuals who have distinct genetic makeups. To do this, they grew embryoid bodies made from human stem cells from different individuals to examine which genes switched on and off as the stem cells that formed the embryoid bodies differentiated into different types of cells. The results showed that it was possible to grow embryoid bodies derived from genetically distinct individuals that consistently produce diverse cell types, similar to those found during human fetal development. Rhodes, Barr et al.’s findings suggest that embryoid bodies are a useful model to study gene regulation across individuals with different genetic backgrounds. This could accelerate research into how genetics are associated with disease by capturing gene regulatory dynamics at an unprecedentedly high spatial and temporal resolution. Additionally, embryoid bodies could be used to explore how exposure to different environmental factors during early development affect disease-related outcomes in adulthood in different individuals.
© 2022, Rhodes et al.
Conflict of interest statement
KR KR is named as an inventor with the University of Chicago on a patent related to the this manuscript (patent pending 63/291,945), KB KAB is named as an inventor with the University of Chicago on a patent related to the this manuscript (patent pending 63/291,945), JP, BS No competing interests declared, AB AB is a consultant for Third Rock Ventures, LLC and a shareholder in Alphabet, Inc, YG YG is named as an inventor with the University of Chicago on a patent related to the this manuscript (patent pending 63/291,945)
Figures













References
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

