Vasculature-on-a-chip platform with innate immunity enables identification of angiopoietin-1 derived peptide as a therapeutic for SARS-CoV-2 induced inflammation
- PMID: 35142777
- PMCID: PMC9207819
- DOI: 10.1039/d1lc00817j
Vasculature-on-a-chip platform with innate immunity enables identification of angiopoietin-1 derived peptide as a therapeutic for SARS-CoV-2 induced inflammation
Abstract
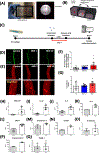
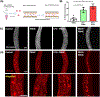
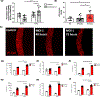
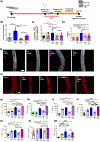
Coronavirus disease 2019 (COVID-19) was primarily identified as a novel disease causing acute respiratory syndrome. However, as the pandemic progressed various cases of secondary organ infection and damage by severe respiratory syndrome coronavirus 2 (SARS-CoV-2) have been reported, including a breakdown of the vascular barrier. As SARS-CoV-2 gains access to blood circulation through the lungs, the virus is first encountered by the layer of endothelial cells and immune cells that participate in host defense. Here, we developed an approach to study SARS-CoV-2 infection using vasculature-on-a-chip. We first modeled the interaction of virus alone with the endothelialized vasculature-on-a-chip, followed by the studies of the interaction of the virus exposed-endothelial cells with peripheral blood mononuclear cells (PBMCs). In an endothelial model grown on a permeable microfluidic bioscaffold under flow conditions, both human coronavirus (HCoV)-NL63 and SARS-CoV-2 presence diminished endothelial barrier function by disrupting VE-cadherin junctions and elevating the level of pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, and angiopoietin-2. Inflammatory cytokine markers were markedly more elevated upon SARS-CoV-2 infection compared to HCoV-NL63 infection. Introduction of PBMCs with monocytes into the vasculature-on-a-chip upon SARS-CoV-2 infection further exacerbated cytokine-induced endothelial dysfunction, demonstrating the compounding effects of inter-cellular crosstalk between endothelial cells and monocytes in facilitating the hyperinflammatory state. Considering the harmful effects of SARS-CoV-2 on endothelial cells, even without active virus proliferation inside the cells, a potential therapeutic approach is critical. We identified angiopoietin-1 derived peptide, QHREDGS, as a potential therapeutic capable of profoundly attenuating the inflammatory state of the cells consistent with the levels in non-infected controls, thereby improving the barrier function and endothelial cell survival against SARS-CoV-2 infection in the presence of PBMC.
Conflict of interest statement
Competing interests
M.R. is a co-founder of TARA Biosystems, she holds equity and receives consulting fees from the company.
Figures

Similar articles
-
Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19.J Biol Regul Homeost Agents. 2020 Sep-Oct,;34(5):1629-1632. doi: 10.23812/20-2EDIT. J Biol Regul Homeost Agents. 2020. PMID: 32945158
-
Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science.Cardiovasc Res. 2020 Dec 1;116(14):2177-2184. doi: 10.1093/cvr/cvaa230. Cardiovasc Res. 2020. PMID: 32750108 Free PMC article. Review.
-
Elevated Cytokine Levels in Plasma of Patients with SARS-CoV-2 Do Not Contribute to Pulmonary Microvascular Endothelial Permeability.Microbiol Spectr. 2022 Feb 23;10(1):e0167121. doi: 10.1128/spectrum.01671-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171047 Free PMC article.
-
Monocyte-crosstalk drives interferon-mediated signaling following SARS-CoV-2 exposure.Mol Syst Biol. 2022 Sep;18(9):e11256. doi: 10.15252/msb.202211256. Mol Syst Biol. 2022. PMID: 36094010 Free PMC article.
-
Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19.Viruses. 2020 Dec 26;13(1):29. doi: 10.3390/v13010029. Viruses. 2020. PMID: 33375371 Free PMC article. Review.
Cited by
-
Microfluidic strategies for biomimetic lung chip establishment and SARS-CoV2 study.Mater Today Bio. 2023 Dec 7;24:100905. doi: 10.1016/j.mtbio.2023.100905. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38094656 Free PMC article. Review.
-
Application of microfluidic technologies on COVID-19 diagnosis and drug discovery.Acta Pharm Sin B. 2023 Feb 24;13(7):2877-96. doi: 10.1016/j.apsb.2023.02.014. Online ahead of print. Acta Pharm Sin B. 2023. PMID: 36855672 Free PMC article. Review.
-
Biomimetic lung-on-a-chip to model virus infection and drug evaluation.Eur J Pharm Sci. 2023 Jan 1;180:106329. doi: 10.1016/j.ejps.2022.106329. Epub 2022 Nov 11. Eur J Pharm Sci. 2023. PMID: 36375766 Free PMC article. Review.
-
Microfluidic Organ-on-A-chip: A Guide to Biomaterial Choice and Fabrication.Int J Mol Sci. 2023 Feb 6;24(4):3232. doi: 10.3390/ijms24043232. Int J Mol Sci. 2023. PMID: 36834645 Free PMC article. Review.
-
SARS-CoV-2 pathogenesis in an angiotensin II-induced heart-on-a-chip disease model and extracellular vesicle screening.Proc Natl Acad Sci U S A. 2024 Jul 9;121(28):e2403581121. doi: 10.1073/pnas.2403581121. Epub 2024 Jul 5. Proc Natl Acad Sci U S A. 2024. PMID: 38968108 Free PMC article.
References
-
- Baig AM, Khaleeq A, Ali U, Syeda H, ACS Chem. Neurosci. 2020, 11, 995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

