Telomeres, aging, and cancer: the big picture
- PMID: 35142846
- PMCID: PMC8832478
- DOI: 10.1182/blood.2021014299
Telomeres, aging, and cancer: the big picture
Abstract
The role of telomeres in human health and disease is yet to be fully understood. The limitations of mouse models for the study of human telomere biology and difficulties in accurately measuring the length of telomere repeats in chromosomes and cells have diverted attention from many important and relevant observations. The goal of this perspective is to summarize some of these observations and to discuss the antagonistic role of telomere loss in aging and cancer in the context of developmental biology, cell turnover, and evolution. It is proposed that both damage to DNA and replicative loss of telomeric DNA contribute to aging in humans, with the differences in leukocyte telomere length between humans being linked to the risk of developing specific diseases. These ideas are captured in the Telomere Erosion in Disposable Soma theory of aging proposed herein.
© 2022 by The American Society of Hematology.
Figures

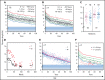
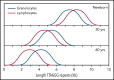

References
-
- Kirkwood TB, Cremer T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum Genet. 1982;60(2):101-121. - PubMed
-
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11(4):398-411.
-
- Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349-352. - PubMed
-
- Kirkwood TB, Holliday R. The evolution of ageing and longevity. Proc R Soc Lond B Biol Sci. 1979;205(1161):531-546. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

