Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination
- PMID: 35143256
- PMCID: PMC8939765
- DOI: 10.1126/science.abn2688
Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination
Abstract
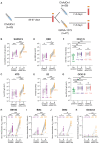
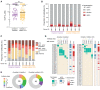
Heterologous prime-boost immunization strategies have the potential to augment COVID-19 vaccine efficacy. We longitudinally profiled severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S)-specific serological and memory B cell (MBC) responses in individuals who received either homologous (ChAdOx1:ChAdOx1) or heterologous (ChAdOx1:mRNA-1273) prime-boost vaccination. Heterologous messenger RNA (mRNA) booster immunization induced higher serum neutralizing antibody and MBC responses against SARS-CoV-2 variants of concern (VOCs) compared with that of homologous ChAdOx1 boosting. Specificity mapping of circulating B cells revealed that mRNA-1273 boost immunofocused ChAdOx1-primed responses onto epitopes expressed on prefusion-stabilized S. Monoclonal antibodies isolated from mRNA-1273-boosted participants displayed overall higher binding affinities and increased breadth of reactivity against VOCs relative to those isolated from ChAdOx1-boosted individuals. Overall, the results provide molecular insight into the enhanced quality of the B cell response induced after heterologous mRNA booster vaccination.
Figures


References
-
- Madhi S. A., Baillie V., Cutland C. L., Voysey M., Koen A. L., Fairlie L., Padayachee S. D., Dheda K., Barnabas S. L., Bhorat Q. E., Briner C., Kwatra G., Ahmed K., Aley P., Bhikha S., Bhiman J. N., Bhorat A. E., du Plessis J., Esmail A., Groenewald M., Horne E., Hwa S.-H., Jose A., Lambe T., Laubscher M., Malahleha M., Masenya M., Masilela M., McKenzie S., Molapo K., Moultrie A., Oelofse S., Patel F., Pillay S., Rhead S., Rodel H., Rossouw L., Taoushanis C., Tegally H., Thombrayil A., van Eck S., Wibmer C. K., Durham N. M., Kelly E. J., Villafana T. L., Gilbert S., Pollard A. J., de Oliveira T., Moore P. L., Sigal A., Izu A.; NGS-SA Group; Wits-VIDA COVID Group , Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 384, 1885–1898 (2021). 10.1056/NEJMoa2102214 - DOI - PMC - PubMed
-
- Hansen C. H., et al., Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study. medRxiv, 2021.2012.2020.21267966 (2021). 10.1101/2021.12.20.21267966 - DOI
-
- Simpson C. R., Shi T., Vasileiou E., Katikireddi S. V., Kerr S., Moore E., McCowan C., Agrawal U., Shah S. A., Ritchie L. D., Murray J., Pan J., Bradley D. T., Stock S. J., Wood R., Chuter A., Beggs J., Stagg H. R., Joy M., Tsang R. S. M., de Lusignan S., Hobbs R., Lyons R. A., Torabi F., Bedston S., O’Leary M., Akbari A., McMenamin J., Robertson C., Sheikh A., First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 27, 1290–1297 (2021). 10.1038/s41591-021-01408-4 - DOI - PMC - PubMed
-
- Schulz J. B., Berlit P., Diener H.-C., Gerloff C., Greinacher A., Klein C., Petzold G. C., Piccininni M., Poli S., Röhrig R., Steinmetz H., Thiele T., Kurth T., German Society of Neurology SARS-CoV-2 Vaccination Study Group , COVID-19 vaccine-associated cerebral venous thrombosis in Germany. Ann. Neurol. 90, 627–639 (2021). 10.1002/ana.26172 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

