Multi-insecticide resistant malaria vectors in the field remain susceptible to malathion, despite the presence of Ace1 point mutations
- PMID: 35143477
- PMCID: PMC8830663
- DOI: 10.1371/journal.pgen.1009963
Multi-insecticide resistant malaria vectors in the field remain susceptible to malathion, despite the presence of Ace1 point mutations
Abstract
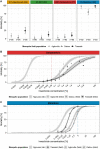
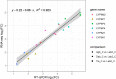
Insecticide resistance in Anopheles mosquitoes is seriously threatening the success of insecticide-based malaria vector control. Surveillance of insecticide resistance in mosquito populations and identifying the underlying mechanisms enables optimisation of vector control strategies. Here, we investigated the molecular mechanisms of insecticide resistance in three Anopheles coluzzii field populations from southern Côte d'Ivoire, including Agboville, Dabou and Tiassalé. All three populations were resistant to bendiocarb, deltamethrin and DDT, but not or only very weakly resistant to malathion. The absence of malathion resistance is an unexpected result because we found the acetylcholinesterase mutation Ace1-G280S at high frequencies, which would typically confer cross-resistance to carbamates and organophosphates, including malathion. Notably, Tiassalé was the most susceptible population to malathion while being the most resistant one to the pyrethroid deltamethrin. The resistance ratio to deltamethrin between Tiassalé and the laboratory reference colony was 1,800 fold. By sequencing the transcriptome of individual mosquitoes, we found numerous cytochrome P450-dependent monooxygenases - including CYP6M2, CYP6P2, CYP6P3, CYP6P4 and CYP6P5 - overexpressed in all three field populations. This could be an indication for negative cross-resistance caused by overexpression of pyrethroid-detoxifying cytochrome P450s that may activate pro-insecticides, thereby increasing malathion susceptibility. In addition to the P450s, we found several overexpressed carboxylesterases, glutathione S-transferases and other candidates putatively involved in insecticide resistance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- WHO. World malaria report 2020. Geneva: World Health Organization (WHO), 2020 Licence: CC BY-NC-SA 3.0 IGO.
-
- WHO. Global plan for insecticide resistance management in malaria vectors. Geneva: World Health Organization (WHO), 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

