Evaluation of antimicrobial photodynamic therapy with acidic methylene blue for the treatment of experimental periodontitis
- PMID: 35143492
- PMCID: PMC8830666
- DOI: 10.1371/journal.pone.0263103
Evaluation of antimicrobial photodynamic therapy with acidic methylene blue for the treatment of experimental periodontitis
Abstract
Objective: To investigate the security and effectiveness of antimicrobial photodynamic therapy (aPDT) with a citric acid-based methylene blue (MB) on the periodontal repair following the treatment of ligature-induced experimental periodontitis (EP) in rats.
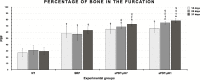
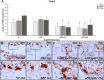
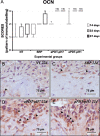
Material and methods: Were used 120 male rats, randomly divided into 4 experimental groups (n = 30): no treatment (NT), SRP alone (SRP), SRP plus aPDT using conventional MB pH 7.0 (aPDT-pH7), SRP plus aPDT using acidic MB pH 1.0 (aPDT-pH1). EP was induced at day 0 by the placement of a ligature around the mandibular left first molars. Ten animals per group/period were euthanized at 14, 22 and 37 days. Histopathological, histometric (percentage of bone in the furcation [PBF]) and immunohistochemical (for tartrate-resistant acid phosphatase [TRAP] and osteocalcin [OCN]) analyses were performed. Data were statistically analyzed.
Results: aPDT-pH1 showed the highest PBF as compared with the other treatments. Collectively, tissues' reaction to both dyes were controlled and healthy for the periodontium. Both aPDT protocols reduced the extent and intensity of the local inflammatory response, reduced the alveolar bone resorption, and promoted a better structural arrangement of the connective tissue as compared with SRP. TRAP expression was downregulated while OCN expression was upregulated by aPDT as compared with SRP alone.
Conclusion: Our data implicate that the novel MB pH 1.0 is as safe as the conventional MB for use in aPDT and raises its additional benefit of increasing the amount of alveolar bone in the furcation.
Conflict of interest statement
The author Juliano Milanezi de Almeida declares that has no conflict of interests related to this study. The author Henrique Rinaldi Matheus declares that has no conflict of interests related to this study. The author Breno Edson Sendão Alves declares that has no conflict of interests related to this study. The author David Jonathan Rodrigues Gusman declares that has no conflict of interests related to this study. The author Elisa Mara de Abreu Furquim declares that has no conflict of interests related to this study. The author Maria José Hitomi Nagata declares that has no conflict of interests related to this study. The author Edilson Ervolino declares that has no conflict of interests related to this study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Effect of the concentration of phenothiazine photosensitizers in antimicrobial photodynamic therapy on bone loss and the immune inflammatory response of induced periodontitis in rats.J Periodontal Res. 2014 Oct;49(5):584-94. doi: 10.1111/jre.12138. Epub 2013 Nov 9. J Periodontal Res. 2014. PMID: 24206053
-
Low-level laser and antimicrobial photodynamic therapy on experimental periodontitis in rats submitted to chemotherapy by 5-fluorouracil.Support Care Cancer. 2017 Oct;25(10):3261-3271. doi: 10.1007/s00520-017-3738-0. Epub 2017 May 9. Support Care Cancer. 2017. PMID: 28488051
-
Effects of butyl toluidine blue photosensitizer on antimicrobial photodynamic therapy for experimental periodontitis treatment in rats.Photodiagnosis Photodyn Ther. 2020 Sep;31:101868. doi: 10.1016/j.pdpdt.2020.101868. Epub 2020 Jun 8. Photodiagnosis Photodyn Ther. 2020. PMID: 32526374
-
Effects of Antimicrobial Photosensitizers of Photodynamic Therapy (PDT) to Treat Periodontitis.Curr Pharm Biotechnol. 2024;25(10):1209-1229. doi: 10.2174/1389201024666230720104516. Curr Pharm Biotechnol. 2024. PMID: 37475551 Review.
-
How effective is adjunctive antimicrobial photodynamic therapy in treating deep periodontal pockets in periodontal disease? A systematic review.J Investig Clin Dent. 2018 Nov;9(4):e12345. doi: 10.1111/jicd.12345. Epub 2018 Jun 4. J Investig Clin Dent. 2018. PMID: 29863310
Cited by
-
TRPC expression in human periodontal ligament cells and the periodontal tissue of periodontitis mice: a preliminary study.Lab Anim Res. 2023 Sep 1;39(1):19. doi: 10.1186/s42826-023-00171-6. Lab Anim Res. 2023. PMID: 37653550 Free PMC article.
-
DNA-aptamer-nanographene oxide as a targeted bio-theragnostic system in antimicrobial photodynamic therapy against Porphyromonas gingivalis.Sci Rep. 2022 Jul 16;12(1):12161. doi: 10.1038/s41598-022-16310-3. Sci Rep. 2022. PMID: 35842460 Free PMC article.
-
Benefits of Antimicrobial Photodynamic Therapy as an Adjunct to Non-Surgical Periodontal Treatment in Smokers with Periodontitis: A Systematic Review and Meta-Analysis.Medicina (Kaunas). 2023 Mar 30;59(4):684. doi: 10.3390/medicina59040684. Medicina (Kaunas). 2023. PMID: 37109642 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

