Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial
- PMID: 35143644
- PMCID: PMC9198908
- DOI: 10.1182/bloodadvances.2021005847
Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial
Abstract
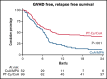
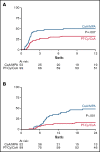
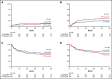
Graft-versus-host disease (GVHD) is the most important complication of allogeneic hematopoietic stem cell transplantation (alloHSCT). We performed a prospective randomized, multicenter, phase 3 trial to study whether posttransplant cyclophosphamide (PT-Cy) combined with a short course of cyclosporine A (CsA) would result in a reduction of severe GVHD and improvement of GVHD-free, relapse-free survival (GRFS) as compared with the combination of CsA and mycophenolic acid (MPA) after nonmyeloablative (NMA) matched related and unrelated peripheral blood alloHSCT. Between October 2013 and June 2018, 160 patients diagnosed with a high-risk hematological malignancy and having a matched related or at least 8 out of 8 HLA-matched unrelated donor were randomized and allocated in a 1:2 ratio to CsA/MPA or PT-Cy/CsA; a total of 151 patients were transplanted (52 vs 99 patients, respectively). The cumulative incidence of grade 2 to 4 acute GVHD at 6 months was 48% in recipients of CsA/MPA vs 30% following PT-Cy/CsA (hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.29-0.82; P = .007). The 2-year cumulative incidence of extensive chronic GVHD was 48% vs 16% (HR, 0.36; 95% CI, 0.21-0.64; P < .001). The 1-year estimate of GRFS was 21% (11% to 32%) vs 45% (35% to 55%), P < .001. With a median follow-up of 56.4 months, relapse incidence, progression-free survival, and overall survival were not significantly different between the 2 treatment arms. PT-Cy combined with a short course of CsA after NMA matched alloHSCT significantly improves GRFS due to a significant reduction in severe acute and chronic GVHD.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Storb R, Deeg HJ, Whitehead J, et al. . Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314(12):729-735. - PubMed
-
- Finke J, Bethge WA, Schmoor C, et al. ; ATG-Fresenius Trial Group . Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855-864. - PubMed
-
- Kröger N, Solano C, Wolschke C, et al. . Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374(1):43-53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

