Humoral and cellular response to COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases under real-life conditions
- PMID: 35143648
- PMCID: PMC8903382
- DOI: 10.1093/rheumatology/keac089
Humoral and cellular response to COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases under real-life conditions
Abstract
Objectives: Successful vaccination is key to overcoming the COVID-19 pandemic. Immunosuppressive medication is known to potentially compromise vaccination responses, and expansion of our knowledge on vaccination efficacy in patients with autoimmune inflammatory rheumatic diseases (AIIRD) is therefore of utmost importance.
Methods: We conducted a single-centre observational study and evaluated the efficacy of approved COVID-19 vaccines in 303 adult AIIRD patients. Serum levels of IgG antibodies against the S1 subunit of SARS-CoV-2 spike proteins (anti-S IgG) were measured at least two weeks after vaccination. In a subgroup of patients without humoral response, T-cell responses were determined using an interferon-γ gamma release assay.
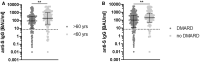
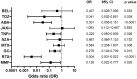
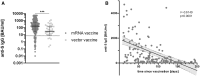
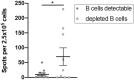
Results: Overall seropositivity rate was 78.5% and was significantly lower in patients under immunosuppressive therapy (75.7 vs 93.2%, P = 0.009). No difference regarding the vaccination type was observed. Glucocorticoids, mycophenolate-mofetil, TNF inhibitors, tocilizumab, abatacept and rituximab were all associated with non-response after proper vaccination. The risk was highest under RTX therapy (OR 0.004, 95% CI 0.001, 0.023, P < 0.0001). A strong negative correlation was observed between time since vaccination with an mRNA vaccine and anti-S antibody levels (r=-0.6149, P < 0.0001). In patients without humoral response, a T-cell response was found in 50%.
Conclusions: COVID-19 vaccination in patients with AIIRD is effective using any approved vaccine. Humoral response might be impaired depending on the individual immunosuppressive medication. The risk of non-response is highest under rituximab therapy. Anti-S IgG antibody levels wane over time after mRNA vaccination. Importantly, 50% of humoral non-responders showed a T-cellular response, suggesting T-cell-mediated protection to a certain extent.
Keywords: COVID-19; cellular T-cell response; humoral response; immunosuppression; rheumatic diseases; vaccination.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study.Ann Rheum Dis. 2021 Oct;80(10):1330-1338. doi: 10.1136/annrheumdis-2021-220647. Epub 2021 Jun 14. Ann Rheum Dis. 2021. PMID: 34127481
-
Efficacy of COVID-19 mRNA vaccination in patients with autoimmune disorders: humoral and cellular immune response.BMC Med. 2023 Jun 14;21(1):210. doi: 10.1186/s12916-023-02868-w. BMC Med. 2023. PMID: 37316832 Free PMC article.
-
B Cell Numbers Predict Humoral and Cellular Response Upon SARS-CoV-2 Vaccination Among Patients Treated With Rituximab.Arthritis Rheumatol. 2022 Jun;74(6):934-947. doi: 10.1002/art.42060. Epub 2022 Apr 17. Arthritis Rheumatol. 2022. PMID: 34962360 Free PMC article.
-
COVID-19 Vaccination in Patients with Autoimmune Inflammatory Rheumatic Diseases: Clinical Guidance of the Korean College of Rheumatology.J Korean Med Sci. 2021 Mar 29;36(12):e95. doi: 10.3346/jkms.2021.36.e95. J Korean Med Sci. 2021. PMID: 33783147 Free PMC article. Review.
-
The Use of COVID-19 Vaccines in Patients with SLE.Curr Rheumatol Rep. 2021 Nov 12;23(11):79. doi: 10.1007/s11926-021-01046-2. Curr Rheumatol Rep. 2021. PMID: 34767100 Free PMC article. Review.
Cited by
-
Complete (Humoral and Cellular) Response to Vaccination against COVID-19 in a Group of Healthcare Workers-Assessment of Factors Affecting Immunogenicity.Vaccines (Basel). 2022 Apr 30;10(5):710. doi: 10.3390/vaccines10050710. Vaccines (Basel). 2022. PMID: 35632467 Free PMC article.
-
Impact of Anti-TNFα Treatment on the Humoral Response to the BNT162b2 mRNA COVID-19 Vaccine in Pediatric Inflammatory Bowel Disease Patients.Vaccines (Basel). 2022 Sep 27;10(10):1618. doi: 10.3390/vaccines10101618. Vaccines (Basel). 2022. PMID: 36298483 Free PMC article.
-
Antibody responses following the surge of SARS-CoV-2 Omicron infection among patients with systemic autoimmune rheumatic diseases.Rheumatol Adv Pract. 2023 Jul 24;7(2):rkad064. doi: 10.1093/rap/rkad064. eCollection 2023. Rheumatol Adv Pract. 2023. PMID: 37547578 Free PMC article.
-
The immune response to Covid-19 mRNA vaccination among Lymphoma patients receiving anti-CD20 treatment.Front Immunol. 2024 Sep 4;15:1433442. doi: 10.3389/fimmu.2024.1433442. eCollection 2024. Front Immunol. 2024. PMID: 39295862 Free PMC article.
-
COVID-19 and rheumatic diseases: A mini-review.Front Med (Lausanne). 2022 Sep 26;9:997876. doi: 10.3389/fmed.2022.997876. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36226148 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

