A New Gene Family Diagnostic for Intracellular Biomineralization of Amorphous Ca Carbonates by Cyanobacteria
- PMID: 35143662
- PMCID: PMC8890360
- DOI: 10.1093/gbe/evac026
A New Gene Family Diagnostic for Intracellular Biomineralization of Amorphous Ca Carbonates by Cyanobacteria
Abstract
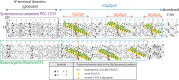
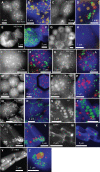
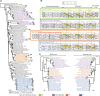
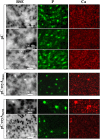
Cyanobacteria have massively contributed to carbonate deposition over the geological history. They are traditionally thought to biomineralize CaCO3 extracellularly as an indirect byproduct of photosynthesis. However, the recent discovery of freshwater cyanobacteria-forming intracellular amorphous calcium carbonates (iACC) challenges this view. Despite the geochemical interest of such a biomineralization process, its molecular mechanisms and evolutionary history remain elusive. Here, using comparative genomics, we identify a new gene (ccyA) and protein family (calcyanin) possibly associated with cyanobacterial iACC biomineralization. Proteins of the calcyanin family are composed of a conserved C-terminal domain, which likely adopts an original fold, and a variable N-terminal domain whose structure allows differentiating four major types among the 35 known calcyanin homologs. Calcyanin lacks detectable full-length homologs with known function. The overexpression of ccyA in iACC-lacking cyanobacteria resulted in an increased intracellular Ca content. Moreover, ccyA presence was correlated and/or colocalized with genes involved in Ca or HCO3- transport and homeostasis, supporting the hypothesis of a functional role of calcyanin in iACC biomineralization. Whatever its function, ccyA appears as diagnostic of intracellular calcification in cyanobacteria. By searching for ccyA in publicly available genomes, we identified 13 additional cyanobacterial strains forming iACC, as confirmed by microscopy. This extends our knowledge about the phylogenetic and environmental distribution of cyanobacterial iACC biomineralization, especially with the detection of multicellular genera as well as a marine species. Moreover, ccyA was probably present in ancient cyanobacteria, with independent losses in various lineages that resulted in a broad but patchy distribution across modern cyanobacteria.
Keywords: amorphous calcium carbonates; biomineralization; cyanobacteria; glycine zipper motifs, comparative genomics; phylogeny; protein structure prediction.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures


References
-
- Aizenberg J, Lambert G, Weiner S, Addadi L.. 2002. Factors involved in the formation of amorphous and crystalline calcium carbonate: a study of an ascidian skeleton. J Am Chem Soc. 124(1):32–39. - PubMed
-
- Altermann W, Kazmierczak J, Oren A, Wright DT.. 2006. Cyanobacterial calcification and its rock-building potential during 3.5 billion years of Earth history. Geobiology 4(3):147–166.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

