Hepatoprotective effects of semaglutide, lanifibranor and dietary intervention in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH
- PMID: 35143711
- PMCID: PMC9099137
- DOI: 10.1111/cts.13235
Hepatoprotective effects of semaglutide, lanifibranor and dietary intervention in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH
Abstract
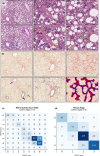
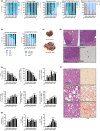
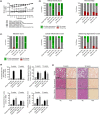
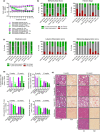
Non-alcoholic steatohepatitis (NASH) has emerged as a major challenge for public health because of high global prevalence and lack of evidence-based therapies. Most animal models of NASH lack sufficient validation regarding disease progression and pharmacological treatment. The Gubra-Amylin NASH (GAN) diet-induced obese (DIO) mouse demonstrate clinical translatability with respect to disease etiology and hallmarks of NASH. This study aimed to evaluate disease progression and responsiveness to clinically effective interventions in GAN DIO-NASH mice. Disease phenotyping was performed in male C57BL/6J mice fed the GAN diet high in fat, fructose, and cholesterol for 28-88 weeks. GAN DIO-NASH mice with biopsy-confirmed NASH and fibrosis received low-caloric dietary intervention, semaglutide (30 nmol/kg/day, s.c.) or lanifibranor (30 mg/kg/day, p.o.) for 8 and 12 weeks, respectively. Within-subject change in nonalcoholic fatty liver disease (NAFLD) Activity Score (NAS) and fibrosis stage was evaluated using automated deep learning-based image analysis. GAN DIO-NASH mice showed clear and reproducible progression in NASH, fibrosis stage, and tumor burden with high incidence of hepatocellular carcinoma. Consistent with clinical trial outcomes, semaglutide and lanifibranor improved NAS, whereas only lanifibranor induced regression in the fibrosis stage. Dietary intervention also demonstrated substantial benefits on metabolic outcomes and liver histology. Differential therapeutic efficacy of semaglutide, lanifibranor, and dietary intervention was supported by quantitative histology, RNA sequencing, and blood/liver biochemistry. In conclusion, the GAN DIO-NASH mouse model recapitulates various histological stages of NASH and faithfully reproduces histological efficacy profiles of compounds in advanced clinical development for NASH. Collectively, these features highlight the utility of GAN DIO-NASH mice in preclinical drug development.
© 2022 Gubra. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.B.M., K.T.T., D.O., A.O., C.G.S., M.R.M., M.F., and H.H.H. are employed by Gubra. S.S.V. was employed by Gubra and is presently employed by Novo Nordisk. L.P., E.S., and A.B. are employed by Boehringer Ingelheim Pharma GmbH & Co. KG. M.V. is employed by Aalborg University, Copenhagen, Denmark. N.V. and J.J. are owners of Gubra. This study was supported by Boehringer‐Ingelheim Pharma. No other potential conflicts of interest relevant to this article were reported.
Figures



References
-
- Yki‐Järvinen H. Non‐alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901‐910. - PubMed
-
- Younossi ZM. Non‐alcoholic fatty liver disease – a global public health perspective. J Hepatol. 2019;70:531‐544. - PubMed
-
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537‐2564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

