Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study
- PMID: 35143771
- PMCID: PMC8820960
- DOI: 10.1016/S2352-4642(22)00004-9
Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study
Abstract
Background: Many adolescents have been affected by the COVID-19 pandemic either directly by being infected with the virus or indirectly by lockdowns and restrictions influencing normal living. We aimed to investigate health, including symptoms of long COVID, in adolescents (aged 15-18 years) who tested positive for SARS-CoV-2 compared with a control group.
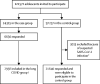
Methods: LongCOVIDKidsDK was a national, cross-sectional study carried out in Denmark, which included SARS-CoV-2-positive adolescents and matched controls. All Danish adolescents aged 15-18 years with a positive SARS-CoV-2 test during the period Jan 1, 2020, to July 12, 2021, and a control group matched (1:4) by age and sex were sent a survey from July 20, 2021. Participants had until Sept 15, 2021, to respond. Symptoms associated with COVID-19, school attendance, and health-related quality of life were investigated using ancillary questions and validated questionnaires (Paediatric Quality of Life Inventory [PedsQL] and Children's Somatic Symptoms Inventory-24 [CSSI-24]). Statistical analyses included descriptive statistics and logistic regression. This study is registered at ClinicalTrials.gov, NCT04786353.
Findings: 24 315 adolescents with a positive SARS-CoV-2 test (case group) and 97 257 matched controls were invited to participate. 3013 matched controls were excluded because of suspected SARS-CoV-2 infection. 6630 (27·3%) responded in the case group and 21 640 (22·3%) responded and were eligible to participate in the control group. Across both groups, median age was 17·6 years (IQR 16·4-18·5), 16 277 (57·6%) of 28 270 responders were female, and 11 993 (42·4%) were male. Participants in the case group had greater odds of having at least one long COVID symptom lasting at least 2 months compared with the control group (3159 [61·9%] vs 12 340 [57·0%], odds ratio 1·22 [95% CI 1·15-1·30]; p<0·0001). Participants in the case group reported significantly lower symptom scores (ie, less somatic distress) on the CSSI-24 than in the control group: mean 10·7 (SD 11·4, median 7·0 [IQR 2·0-15·0]) versus 11·9 (10·6, 9·0 [4·0-17·0]; p<0·0001). Participants in the case group had better quality of life scores on the PedsQL than in the control group: physical functioning mean score 88·7 (SD 13·9, median 93·8 [IQR 84·4-100·0]) versus 86·5 (14·3, 90·6 [81·3-96·9]; p<0·0001); emotional functioning 77·1 (20·3, 80·0 [65·0-95·0]) versus 71·7 (21·4, 75·0 [60·0-90·0]; p<0·0001); social functioning 93·1 (12·5, 100·0 [90·0-100·0]) versus 88·4 (16·2, 95·0 [80·0-100·0]; p<0·0001); and school functioning 66·9 (22·5, 65·0 [60·0-85·0]) versus 62·9 (22·1, 65·0 [50·0-80·0]; p<0·0001). More participants in the case group than in the control group reported 16 or more sick days (1205 [18·2%] vs 2518 [11·6%]; p<0·0001) and 16 or more days of school absence (695 [10·5%] vs 1777 [8·2%]; p<0·0001).
Interpretation: Participants with SARS-CoV-2-positive tests had more long-lasting symptoms and sick leave, whereas participants in the control group had more short-lasting symptoms and worse quality of life. Knowledge of long COVID in adolescents is important to guide clinical recognition and management of this condition.
Funding: AP Møller and Chastine McKinney Møller Foundation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests SDN received a research grant from Novo Nordisk Foundation, a travel grant from Gilead, and is on the advisory board for Gilead, GSK, and MSD. All other authors declare no competing interests.
Figures

References
-
- WHO A clinical case definition of post COVID-19 condition by a Delphi consensus. 6 October 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_cond... - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

