Senescence and the tumor-immune landscape: Implications for cancer immunotherapy
- PMID: 35143990
- PMCID: PMC9357237
- DOI: 10.1016/j.semcancer.2022.02.005
Senescence and the tumor-immune landscape: Implications for cancer immunotherapy
Abstract
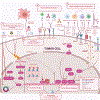
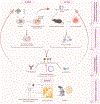
Cancer therapies, including conventional chemotherapy, radiation, and molecularly targeted agents, can lead to tumor eradication through a variety of mechanisms. In addition to their effects on tumor cell growth and survival, these regimens can also influence the surrounding tumor-immune microenvironment in ways that ultimately impact therapy responses. A unique biological outcome of cancer therapy is induction of cellular senescence. Senescence is a damage-induced stress program that leads to both the durable arrest of tumor cells and remodeling the tumor-immune microenvironment through activation of a collection pleiotropic cytokines, chemokines, growth factors, and proteinases known as the senescence-associated secretory phenotype (SASP). Depending on the cancer context and the mechanism of action of the therapy, the SASP produced following therapy-induced senescence (TIS) can promote anti-tumor immunity that enhances therapeutic efficacy, or alternatively chronic inflammation that leads to therapy failure and tumor relapse. Thus, a deeper understanding of the mechanisms regulating the SASP and components necessary for robust anti-tumor immune surveillance in different cancer and therapy contexts are key to harnessing senescence for tumor control. Here we draw a roadmap to modulate TIS and its immune-stimulating features for cancer immunotherapy.
Keywords: Cellular senescence; Immunotherapy; Senescence-associated secretory phenotype; Senotherapeutics; Tumor microenvironment.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors report no declarations of interest.
Figures
References
-
- Galluzzi L, Zitvogel L, Kroemer G, Immunological Mechanisms Underneath the Efficacy of Cancer Therapy, Cancer Immunol Res 4(11) (2016) 895–902. - PubMed
-
- Sherr CJ, Bartek J, Cell Cycle–Targeted Cancer Therapies, Annual Review of Cancer Biology 1(1) (2017) 41–57.
-
- Rixe O, Fojo T, Is Cell Death a Critical End Point for Anticancer Therapies or Is Cytostasis Sufficient?, Clinical Cancer Research 13(24) (2007) 7280–7287. - PubMed
-
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G, Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018, Cell Death Differ 25(3) (2018) 486–541. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

