Diagnostic criteria of perfluorobutane-enhanced ultrasonography for diagnosing hepatocellular carcinoma in high-risk individuals: how is late washout determined?
- PMID: 35144328
- PMCID: PMC9262666
- DOI: 10.14366/usg.21172
Diagnostic criteria of perfluorobutane-enhanced ultrasonography for diagnosing hepatocellular carcinoma in high-risk individuals: how is late washout determined?
Abstract
Purpose: The aim of this study was to investigate the optimal washout criteria of perfluorobutane-enhanced ultrasonography (PFB-US) for the diagnosis of hepatocellular carcinoma (HCC) in high-risk individuals.
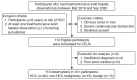
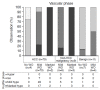
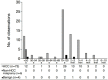
Methods: Participants at risk of HCC with treatment-naïve solid hepatic observations (≥1 cm) who underwent PFB-US from March 2019 to September 2020 were prospectively recruited. Arterial phase hyperenhancement (APHE), washout time, and washout degree were evaluated. The diagnosis of HCC was made by non-rim APHE with late and mild washout. The per-lesion diagnostic performance for diagnosing HCC using different cutoffs for late washout (50, 55, 60, 65, and 70 seconds postcontrast) and the different time windows for determining washout (until 2, 3, 4, 5, 6, 7, 8, 9, and 10 minutes postcontrast) were compared using the McNemar test.
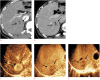
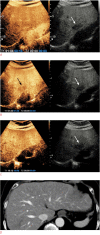
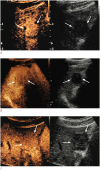
Results: In total, 101 participants with 113 observations (mean size, 33.5±2.8 mm; HCCs [n=82], non-HCC malignancies [n=16], benign [n=15]) were evaluated. Non-rim APHE was observed in 86.6% (71/82) of HCCs. As the cutoff time for late washout increased, the specificity increased to 100% (95% confidence interval [CI], 88.8% to 100%) at the 60-second cutoff with 62.2% sensitivity (95% CI, 50.8% to 72.7%). When the time window for determining washout became wider, the sensitivity and accuracy increased until 6 minutes, with 100% specificity at all times.
Conclusion: Determining washout within 6 minutes after contrast injection with a 60-second cutoff for late washout showed the highest sensitivity without losing specificity for diagnosing HCC using PFB-US in individuals at high risk.
Keywords: Contrast-enhanced ultrasonography; Hepatocellular carcinoma; Perfluorobutane.
Conflict of interest statement
This study was supported by a research grant from Samsung Medison (Seoul, Korea).
Figures
References
-
- American College of Radiology . Reston, VA: American College of Radiology; 2017. CEUS LI-RADS V2017 [Internet] [ctied 2021 Aug 19]. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RAD....
-
- Schwarze V, Marschner C, Negrao de Figueiredo G, Rubenthaler J, Clevert DA. Single-center study: evaluating the diagnostic performance and safety of contrast-enhanced ultrasound (CEUS) in pregnant women to assess hepatic lesions. Ultraschall Med. 2020;41:29–35. - PubMed
-
- Yusuf GT, Sellars ME, Deganello A, Cosgrove DO, Sidhu PS. Retrospective analysis of the safety and cost implications of pediatric contrast-enhanced ultrasound at a single center. AJR Am J Roentgenol. 2017;208:446–452. - PubMed
-
- Kang HJ, Kim JH, Joo I, Han JK. Additional value of contrast-enhanced ultrasound (CEUS) on arterial phase non-hyperenhancement observations (≥ 2 cm) of CT/MRI for high-risk patients: focusing on the CT/MRI LI-RADS categories LR-3 and LR4. Abdom Radiol (NY) 2020;45:55–63. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

