Clinical pharmacokinetics of quinine and its relationship with treatment outcomes in children, pregnant women, and elderly patients, with uncomplicated and complicated malaria: a systematic review
- PMID: 35144612
- PMCID: PMC8832728
- DOI: 10.1186/s12936-022-04065-1
Clinical pharmacokinetics of quinine and its relationship with treatment outcomes in children, pregnant women, and elderly patients, with uncomplicated and complicated malaria: a systematic review
Abstract
Background: Standard dosage regimens of quinine formulated for adult patients with uncomplicated and complicated malaria have been applied for clinical uses in children, pregnant women, and elderly. Since these populations have anatomical and physiological differences from adults, dosage regimens formulated for adults may not be appropriate. The study aimed to (i) review existing information on the pharmacokinetics of quinine in children, pregnant women, and elderly populations, (ii) identify factors that influence quinine pharmacokinetics, and (iii) analyse the relationship between the pharmacokinetics and treatment outcomes (therapeutic and safety) of various dosage regimens of quinine.
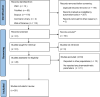
Methods: Web of Sciences, Cochrane Library, Scopus, and PubMed were the databases applied in this systematic search for relevant research articles published up to October 2020 using the predefined search terms. The retrieved articles were initially screened by titles and abstracts to exclude any irrelevant articles and were further evaluated based on full-texts, applying the predefined eligibility criteria. Excel spreadsheet (Microsoft, WA, USA) was used for data collection and management. Qualitative data are presented as numbers and percentages, and where appropriate, mean + SD or median (range) or range values.
Results: Twenty-eight articles fulfilled the eligibility criteria, 19 in children, 7 in pregnant women, and 2 in elderly (14 and 7 articles in complicated and uncomplicated malaria, respectively). Severity of infection, routes of administration, and nutritional status were shown to be the key factors impacting quinine pharmacokinetics in these vulnerable groups.
Conclusions: The recommended dosages for both uncomplicated and complicated malaria are, in general, adequate for elderly and children with uncomplicated malaria. Dose adjustment may be required in pregnant women with both uncomplicated and complicated malaria, and in children with complicated malaria. Pharmacokinetics studies relevant to clinical efficacy in these vulnerable groups of patients with large sample size and reassessment of MIC (minimum inhibitory concentration) should be considered.
Keywords: Children; Elderly; Pharmacokinetics; Pregnancy; Quinine; Systematic-review.
© 2022. The Author(s).
Conflict of interest statement
Authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures
Similar articles
-
UK malaria treatment guidelines.J Infect. 2007 Feb;54(2):111-21. doi: 10.1016/j.jinf.2006.12.003. Epub 2007 Jan 9. J Infect. 2007. PMID: 17215045
-
Population pharmacokinetics of quinine in pregnant women with uncomplicated Plasmodium falciparum malaria in Uganda.J Antimicrob Chemother. 2014 Nov;69(11):3033-40. doi: 10.1093/jac/dku228. Epub 2014 Jun 25. J Antimicrob Chemother. 2014. PMID: 24970740 Free PMC article. Clinical Trial.
-
Efficacy of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: a protocol for systematic review and individual patient data (IPD) meta-analysis.BMJ Open. 2019 Aug 22;9(8):e027503. doi: 10.1136/bmjopen-2018-027503. BMJ Open. 2019. PMID: 31444179 Free PMC article.
-
Artemether for severe malaria.Cochrane Database Syst Rev. 2019 Jun 18;6(6):CD010678. doi: 10.1002/14651858.CD010678.pub3. Cochrane Database Syst Rev. 2019. PMID: 31210357 Free PMC article.
-
Treatment regimens for pregnant women with falciparum malaria.Expert Rev Anti Infect Ther. 2016 Aug;14(8):691-704. doi: 10.1080/14787210.2016.1202758. Epub 2016 Jul 6. Expert Rev Anti Infect Ther. 2016. PMID: 27322015 Review.
Cited by
-
Plasmodium falciparum quinine resistance is multifactorial and includes a role for the drug/metabolite transporters PfCRT and DMT1.bioRxiv [Preprint]. 2025 Apr 11:2024.09.27.615529. doi: 10.1101/2024.09.27.615529. bioRxiv. 2025. PMID: 39386571 Free PMC article. Preprint.
-
Diagnosis and management of malaria in the intensive care unit.J Intensive Med. 2023 Nov 3;4(1):3-15. doi: 10.1016/j.jointm.2023.09.002. eCollection 2024 Jan. J Intensive Med. 2023. PMID: 38263976 Free PMC article. Review.
-
Updates in central nervous system malaria: literature review and considerations.Curr Opin Infect Dis. 2022 Jun 1;35(3):255-261. doi: 10.1097/QCO.0000000000000829. Curr Opin Infect Dis. 2022. PMID: 35665720 Free PMC article. Review.
References
-
- WHO. Guidelines for the treatment of malaria. 3rd Edn. Geneva, World Health Organization, 2015. - PubMed
-
- Saito M, Mansoor R, Kennon K, Anvikar AR, Ashley EA, Chandramohan D, et al. Efficacy and tolerability of artemisinin-based and quinine-based treatments for uncomplicated falciparum malaria in pregnancy: a systematic review and individual patient data meta-analysis. Lancet Infect Dis. 2020;20:943–952. - PMC - PubMed
-
- Anger GJ, Piquette-Miller M. Pharmacokinetic studies in pregnant women. Clin Pharmacol Ther. 2008;83:184–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical