Effect of indacaterol/glycopyrronium on ventilation and perfusion in COPD: a randomized trial
- PMID: 35144620
- PMCID: PMC8832861
- DOI: 10.1186/s12931-022-01949-3
Effect of indacaterol/glycopyrronium on ventilation and perfusion in COPD: a randomized trial
Abstract
Rationale: The long-acting β2-agonist/long-acting muscarinic antagonist combination indacaterol/glycopyrronium (IND/GLY) elicits bronchodilation, improves symptoms, and reduces exacerbations in COPD. Magnetic resonance imaging (MRI) of the lung with hyperpolarized gas and gadolinium contrast enhancement enables assessment of whole lung functional responses to IND/GLY.
Objectives: The primary objective was assessment of effect of IND/GLY on global ventilated lung volume (%VV) versus placebo in COPD. Lung function, regional ventilation and perfusion in response to IND/GLY were also measured.
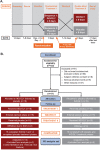
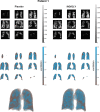
Methods: This double-blind, randomized, placebo-controlled, crossover study assessed %VV and pulmonary perfusion in patients with moderate-to-severe COPD after 8 days of once-daily IND/GLY treatment (110/50 µg) followed by 8 days of placebo, or vice versa, using inhaled hyperpolarized 3He gas and gadolinium contrast-enhanced MRI, respectively. Lung function measures including spirometry were performed for each treatment after 8 days.
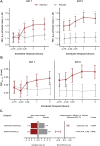
Measurements and main results: Of 31 patients randomized, 29 completed both treatment periods. IND/GLY increased global %VV versus placebo (61.73% vs. 56.73%, respectively, least squares means treatment difference: 5.00% [90% CI 1.40 to 8.60]; P = 0.025). IND/GLY improved whole lung index of ventilation volume to perfusion volume (V/Q) ratio versus placebo; 94% (90% CI 83 to 105) versus 86% (90% CI 75 to 97; P = 0.047), respectively. IND/GLY showed a trend to improve diffusing capacity for carbon monoxide (DLCO) (+ 0.66 mL/min/mmHg; P = 0.082). By Day 8, forced expiratory volume in 1 s (FEV1) was increased by 0.32 L versus placebo (90% CI 0.26 to 0.38; P < 0.0001), substantiating earlier findings and providing evidence of assay sensitivity for this trial.
Conclusions: IND/GLY improved lung ventilation assessed by 3He MRI after 1 week of treatment. This observation may provide mechanistic support for the symptomatic clinical benefit shown with IND/GLY in COPD. Clinical trial registered with www.clinicaltrials.gov (NCT02634983).
Keywords: Chronic obstructive pulmonary disease; Hyperpolarized 3He gas magnetic resonance imaging; Indacaterol/glycopyrronium; V/Q index; Ventilation volume and perfusion volume; Ventilation/perfusion ratio.
© 2022. The Author(s).
Conflict of interest statement
Dave Singh reports personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Teva, Theravance, and Verona, outside the submitted work.
Figures

Similar articles
-
Efficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study.Int J Chron Obstruct Pulmon Dis. 2014 Feb 24;9:215-28. doi: 10.2147/COPD.S51592. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 24596459 Free PMC article. Clinical Trial.
-
A randomized trial to determine the impact of indacaterol/glycopyrronium on nighttime oxygenation and symptoms in patients with moderate-to-severe COPD: the DuoSleep study.Int J Chron Obstruct Pulmon Dis. 2019 Jan 9;14:199-210. doi: 10.2147/COPD.S184127. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 30666100 Free PMC article. Clinical Trial.
-
Effects of indacaterol/glycopyrronium (QVA149) on lung hyperinflation and physical activity in patients with moderate to severe COPD: a randomised, placebo-controlled, crossover study (The MOVE Study).BMC Pulm Med. 2016 Jun 14;16(1):95. doi: 10.1186/s12890-016-0256-7. BMC Pulm Med. 2016. PMID: 27301417 Free PMC article. Clinical Trial.
-
Role of dual bronchodilators in COPD: A review of the current evidence for indacaterol/glycopyrronium.Pulm Pharmacol Ther. 2017 Aug;45:19-33. doi: 10.1016/j.pupt.2017.04.002. Epub 2017 Apr 4. Pulm Pharmacol Ther. 2017. PMID: 28389258 Review.
-
Role of combined indacaterol and glycopyrronium bromide (QVA149) for the treatment of COPD in Japan.Int J Chron Obstruct Pulmon Dis. 2015 Apr 21;10:813-22. doi: 10.2147/COPD.S56067. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25960646 Free PMC article. Review.
Cited by
-
From COPD to cancer: indacaterol's unexpected role in combating NSCLC.Front Pharmacol. 2025 Apr 3;16:1579126. doi: 10.3389/fphar.2025.1579126. eCollection 2025. Front Pharmacol. 2025. PMID: 40276602 Free PMC article.
-
Implications of Cardiopulmonary Risk for the Management of COPD: A Narrative Review.Adv Ther. 2024 Jun;41(6):2151-2167. doi: 10.1007/s12325-024-02855-4. Epub 2024 Apr 25. Adv Ther. 2024. PMID: 38664329 Free PMC article. Review.
-
Mortality prevention as the centre of COPD management.ERJ Open Res. 2024 Jun 17;10(3):00850-2023. doi: 10.1183/23120541.00850-2023. eCollection 2024 May. ERJ Open Res. 2024. PMID: 38887682 Free PMC article. Review.
-
Lung functional imaging.Breathe (Sheff). 2023 Sep;19(3):220272. doi: 10.1183/20734735.0272-2022. Epub 2023 Nov 14. Breathe (Sheff). 2023. PMID: 38020338 Free PMC article.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2020 http://goldcopd.org.
-
- Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV 1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12(1):1–9. doi: 10.1186/1465-9921-12-40. - DOI - PMC - PubMed

